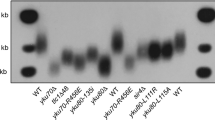
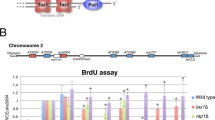
Abstract
Budding yeast telomeres are reversibly bound at the nuclear envelope through two partially redundant pathways that involve the Sir2/3/4 silencing complex and the Yku70/80 heterodimer1,2. To better understand how this is regulated, we studied the role of SUMOylation in telomere anchoring. We find that the PIAS-like SUMO E3 ligase Siz2 sumoylates both Yku70/80 and Sir4 in vivo and promotes telomere anchoring to the nuclear envelope. Remarkably, loss of Siz2 also provokes telomere extension in a telomerase-dependent manner that is epistatic with loss of the helicase Pif1. Consistent with our previously documented role for telomerase in anchorage3, normal telomere anchoring in siz2 Δ is restored by PIF1 deletion. By live-cell imaging of a critically short telomere, we show that telomeres shift away from the nuclear envelope when elongating. We propose that SUMO-dependent association with the nuclear periphery restrains bound telomerase, whereas active elongation correlates with telomere release.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hediger, F., Neumann, F. R., Van Houwe, G., Dubrana, K. & Gasser, S. M. Live imaging of telomeres: yKu and Sir proteins define redundant telomere-anchoring pathways in yeast. Curr. Biol. 12, 2076–2089 (2002).
Palladino, F. et al. SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres. Cell 75, 543–555 (1993).
Schober, H., Ferreira, H., Kalck, V., Gehlen, L. R. & Gasser, S. M. Yeast telomerase and the SUN domain protein Mps3 anchor telomeres and repress subtelomeric recombination. Genes Dev. 23, 928–938 (2009).
Bupp, J. M., Martin, A. E., Stensrud, E. S. & Jaspersen, S. L. Telomere anchoring at the nuclear periphery requires the budding yeast Sad1-UNC-84 domain protein Mps3. J. Cell Biol. 179, 845–854 (2007).
Taddei, A., Hediger, F., Neumann, F. R., Bauer, C. & Gasser, S. M. Separation of silencing from perinuclear anchoring functions in yeast Ku80, Sir4 and Esc1 proteins. EMBO J. 23, 1301–1312 (2004).
Zhao, X., Wu, C. Y. & Blobel, G. Mlp-dependent anchorage and stabilization of a desumoylating enzyme is required to prevent clonal lethality. J. Cell Biol. 167, 605–611 (2004).
Nathan, D. et al. Histone sumoylation is a negative regulator in Saccharomyces cerevisiae and shows dynamic interplay with positive-acting histone modifications. Genes Dev. 20, 966–976 (2006).
Seufert, W., Futcher, B. & Jentsch, S. Role of a ubiquitin-conjugating enzyme in degradation of S- and M-phase cyclins. Nature 373, 78–81 (1995).
Gartenberg, M. R., Neumann, F. R., Laroche, T., Blaszczyk, M. & Gasser, S. M. Sir-mediated repression can occur independently of chromosomal and subnuclear contexts. Cell 119, 955–967 (2004).
Mondoux, M. A., Scaife, J. G. & Zakian, V. A. Differential nuclear localization does not determine the silencing status of Saccharomyces cerevisiae telomeres. Genetics 177, 2019–2029 (2007).
Taddei, A. & Gasser, S. M. Multiple pathways for telomere tethering: functional implications of subnuclear position for heterochromatin formation. Biochim. Biophys. Acta 1677, 120–128 (2004).
Andrulis, E. D. et al. Esc1, a nuclear periphery protein required for Sir4-based plasmid anchoring and partitioning. Mol. Cell. Biol. 22, 8292–8301 (2002).
Ansari, A. & Gartenberg, M. R. The yeast silent information regulator Sir4p anchors and partitions plasmids. Mol. Cell. Biol. 17, 7061–7068 (1997).
Denison, C. et al. A proteomic strategy for gaining insights into protein sumoylation in yeast. Mol. Cell. Proteomics 4, 246–254 (2005).
Hannich, J. T. et al. Defining the SUMO-modified proteome by multiple approaches in Saccharomyces cerevisiae. J. Biol. Chem. 280, 4102–4110 (2005).
Wohlschlegel, J. A., Johnson, E. S., Reed, S. I. & Yates, J. R. III Global analysis of protein sumoylation in Saccharomyces cerevisiae. J. Biol. Chem. 279, 45662–45668 (2004).
Roy, R., Meier, B., McAinsh, A. D., Feldmann, H. M. & Jackson, S. P. Separation-of-function mutants of yeast Ku80 reveal a Yku80p–Sir4p interaction involved in telomeric silencing. J. Biol. Chem. 279, 86–94 (2004).
Zhao, X. & Blobel, G. A SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proc. Natl Acad. Sci. USA 102, 4777–4782 (2005).
Carter, S. & Vousden, K. H. p53-Ubl fusions as models of ubiquitination, sumoylation and neddylation of p53. Cell Cycle 7, 2519–2528 (2008).
Zhu, S., Zhang, H. & Matunis, M. J. SUMO modification through rapamycin-mediated heterodimerization reveals a dual role for Ubc9 in targeting RanGAP1 to nuclear pore complexes. Exp. Cell Res. 312, 1042–1049 (2006).
Johnson, E. S., Schwienhorst, I., Dohmen, R. J. & Blobel, G. The ubiquitin-like protein Smt3p is activated for conjugation to other proteins by an Aos1p/Uba2p heterodimer. EMBO J. 16, 5509–5519 (1997).
Chen, X. L. et al. Topoisomerase I-dependent viability loss in Saccharomyces cerevisiae mutants defective in both SUMO conjugation and DNA repair. Genetics 177, 17–30 (2007).
Hirano, Y., Fukunaga, K. & Sugimoto, K. Rif1 and rif2 inhibit localization of tel1 to DNA ends. Mol. Cell 33, 312–322 (2009).
Boule, J. B., Vega, L. R. & Zakian, V. A. The yeast Pif1p helicase removes telomerase from telomeric DNA. Nature 438, 57–61 (2005).
Teixeira, M. T., Arneric, M., Sperisen, P. & Lingner, J. Telomere length homeostasis is achieved via a switch between telomerase-extendible and -nonextendible states. Cell 117, 323–335 (2004).
Marcand, S., Brevet, V., Mann, C. & Gilson, E. Cell cycle restriction of telomere elongation. Curr. Biol. 10, 487–490 (2000).
Wellinger, R. J., Wolf, A. J. & Zakian, V. A. Saccharomyces telomeres acquire single-strand TG1-3 tails late in S phase. Cell 72, 51–60 (1993).
Xhemalce, B. et al. Role of SUMO in the dynamics of telomere maintenance in fission yeast. Proc. Natl Acad. Sci USA 104, 893–898 (2007).
Ungar, L. et al. A genome-wide screen for essential yeast genes that affect telomere length maintenance. Nucleic Acids Res. 37, 3840–3849 (2009).
Panse, V. G., Kuster, B., Gerstberger, T. & Hurt, E. Unconventional tethering of Ulp1 to the transport channel of the nuclear pore complex by karyopherins. Nat. Cell Biol. 5, 21–27 (2003).
Hiraga, S., Botsios, S. & Donaldson, A. D. Histone H3 lysine 56 acetylationby Rtt109 is crucial for chromosome positioning. J. Cell Biol. 183, 641–651 (2008).
Potts, P. R. & Yu, H. The SMC5/6 complex maintains telomere length in ALT cancer cells through SUMOylation of telomere-binding proteins. Nat. Struct. Mol. Biol. 14, 581–590 (2007).
Nagai, S. et al. Functional targeting of DNA damage to a nuclear pore-associated SUMO-dependent ubiquitin ligase. Science 322, 597–602 (2008).
Kalocsay, M., Hiller, N. J. & Jentsch, S. Chromosome-wide Rad51 spreading and SUMO-H2A.Z-dependent chromosome fixation in response to a persistent DNA double-strand break. Mol. Cell 33, 335–343 (2009).
Galanty, Y. et al. Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature 462, 935–939 (2009).
Morris, J. R. et al. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature 462, 886–890 (2009).
Abdallah, P. et al. A two-step model for senescence triggered by a single critically short telomere. Nat. Cell Biol. 11, 988–993 (2009).
Khadaroo, B. et al. The DNA damage response at eroded telomeres and tethering to the nuclear pore complex. Nat. Cell Biol. 11, 980–987 (2009).
Marvin, M. E. et al. The association of yKu with subtelomeric core X sequences prevents recombination involving telomeric sequences. Genetics 183, 453–467 (2009).
Ribes-Zamora, A., Mihalek, I., Lichtarge, O. & Bertuch, A. A. Distinct faces of the Ku heterodimer mediate DNA repair and telomeric functions. Nat. Struct. Mol. Biol. 14, 301–307 (2007).
Longtine, M. S. et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953–961 (1998).
Parker, R. E. Introductory Statistics for Biology 2nd edn (Cambridge Univ.Press, 1997).
Acknowledgements
We thank V. Zakian and E. Johnson for yeast strains, and N. Iglesias and M. Arneric for advice on the single-telomere extension system. H.C.F. was financially supported by Marie Curie and EMBO long-term fellowships, and the Gasser Laboratory by the Novartis Research Foundation. J.L. was supported by the Swiss National Science Foundation, the European Community’s Seventh Framework Programme (grant 200950) and a European Research Council advanced investigator grant (grant 232812). Both laboratories are part of the SNF-funded Frontiers in Genetics National Center of Competence in Research.
Author information
Authors and Affiliations
Contributions
S.M.G. and J.L. directed the study. H.C.F. did the biochemical experiments and with the help of V.K. carried out all telomere localization and minimal anchor experiments. B.L. did most of the telomere length assays and H.S. did the short-telomere mating assay. The manuscript was prepared by H.C.F. and S.M.G. with contributions from J.L., B.L. and H.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1118 kb)
Supplementary Information
Supplementary Table 1 (XLS 14 kb)
Rights and permissions
About this article
Cite this article
Ferreira, H., Luke, B., Schober, H. et al. The PIAS homologue Siz2 regulates perinuclear telomere position and telomerase activity in budding yeast. Nat Cell Biol 13, 867–874 (2011). https://doi.org/10.1038/ncb2263
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2263
This article is cited by
-
Elevated dosage of Ulp1 disrupts telomeric silencing in Saccharomyces cerevisiae
Molecular Biology Reports (2018)
-
Chromatin states and nuclear organization in development — a view from the nuclear lamina
Genome Biology (2015)
-
Spatial reorganization of telomeres in long-lived quiescent cells
Genome Biology (2015)
-
Sumoylation and transcription regulation at nuclear pores
Chromosoma (2015)
-
End-joining inhibition at telomeres requires the translocase and polySUMO-dependent ubiquitin ligase Uls1
The EMBO Journal (2013)