Abstract
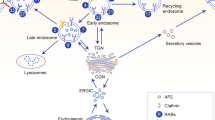
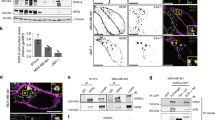
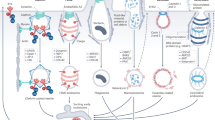
Compartmentalization of signals generated by receptor tyrosine kinase (RTK) endocytosis has emerged as a major determinant of various cell functions. Here, using tumour-associated Met-activating mutations, we demonstrate a direct link between endocytosis and tumorigenicity. Met mutants exhibit increased endocytosis/recycling activity and decreased levels of degradation, leading to accumulation on endosomes, activation of the GTPase Rac1, loss of actin stress fibres and increased levels of cell migration. Blocking endocytosis inhibited mutants’ anchorage-independent growth, in vivo tumorigenesis and metastasis while maintaining their activation. One mutant resistant to inhibition by a Met-specific tyrosine kinase inhibitor was sensitive to endocytosis inhibition. Thus, oncogenicity of Met mutants results not only from activation but also from their altered endocytic trafficking, indicating that endosomal signalling may be a crucial mechanism regulating RTK-dependent tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Teis, D., Wunderlich, W. & Huber, L. A. Localization of the MP1-MAPK scaffold complex to endosomes is mediated by p14 and required for signal transduction. Dev. Cell 3, 803–814 (2002).
Palamidessi, A. et al. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 134, 135–147 (2008).
Schenck, A. et al. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell 133, 486–497 (2008).
McPherson, P. S., Kay, B. K. & Hussain, N. K. Signaling on the endocytic pathway. Traffic 2, 375–384 (2001).
Kermorgant, S., Zicha, D. & Parker, P. J. PKC controls HGF-dependent c-Met traffic, signalling and cell migration. EMBO J. 23, 3721–3734 (2004).
Miaczynska, M., Pelkmans, L. & Zerial, M. Not just a sink: endosomes in control of signal transduction. Curr. Opin. Cell Biol. 16, 400–406 (2004).
Kermorgant, S. & Parker, P. J. Receptor trafficking controls weak signal delivery: a strategy used by c-Met for STAT3 nuclear accumulation. J. Cell Biol. 182, 855–863 (2008).
Sorkin, A., McClure, M., Huang, F. & Carter, R. Interaction of EGF receptor and grb2 in living cells visualized by fluorescence resonance energy transfer (FRET) microscopy. Curr. Biol. 10, 1395–1398 (2000).
Vieira, A. V., Lamaze, C. & Schmid, S. L. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274, 2086–2089 (1996).
Howe, C. L., Valletta, J. S., Rusnak, A. S. & Mobley, W. C. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron 32, 801–814 (2001).
Le Roy, C. & Wrana, J. L. Signaling and endocytosis: a team effort for cell migration. Dev. Cell 9, 167–168 (2005).
Polo, S. & Di Fiore, P. P. Endocytosis conducts the cell signaling orchestra. Cell 124, 897–900 (2006).
von Zastrow, M. & Sorkin, A. Signaling on the endocytic pathway. Curr. Opin. Cell Biol. 19, 436–445 (2007).
Kermorgant, S. & Parker, P. J. c-Met signalling: spatio-temporal decisions. Cell Cycle 4, 352–355 (2005).
Gould, G. W. & Lippincott-Schwartz, J. New roles for endosomes: from vesicular carriers to multi-purpose platforms. Nat. Rev. Mol. Cell Biol. 10, 287–292 (2009).
Jekely, G., Sung, H. H., Luque, C. M. & Rorth, P. Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Dev. Cell 9, 197–207 (2005).
Wang, Y., Pennock, S., Chen, X. & Wang, Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol. Cell Biol. 22, 7279–7290 (2002).
Kholodenko, B. N. Cell-signalling dynamics in time and space. Nat. Rev. Mol. Cell Biol. 7, 165–176 (2006).
Lanzetti, L. & Di Fiore, P. P. Endocytosis and cancer: an ‘insider’ network with dangerous liaisons. Traffic 9, 2011–2021 (2008).
Mosesson, Y., Mills, G. B. & Yarden, Y. Derailed endocytosis: an emerging feature of cancer. Nat. Rev. Cancer 8, 835–850 (2008).
Rao, D. S. et al. Altered receptor trafficking in Huntingtin Interacting Protein 1-transformed cells. Cancer Cell 3, 471–482 (2003).
Comoglio, P. M. Pathway specificity for Met signalling. Nat. Cell Biol. 3, E161–E162 (2001).
Birchmeier, C., Birchmeier, W., Gherardi, E. & Vande Woude, G. F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 4, 915–925 (2003).
Christensen, J. G., Burrows, J. & Salgia, R. c-Met as a target for human cancer and characterization of inhibitors for therapeutic intervention. Cancer Lett. 225, 1–26 (2005).
Schmidt, L. et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 16, 68–73 (1997).
Jeffers, M. et al. Activating mutations for the met tyrosine kinase receptor in human cancer. Proc. Natl Acad. Sci. USA 94, 11445–11450 (1997).
Bardelli, A. et al. Uncoupling signal transducers from oncogenic MET mutants abrogates cell transformation and inhibits invasive growth. Proc. Natl Acad. Sci. USA 95, 14379–14383 (1998).
Graveel, C. R., London, C. A. & Vande Woude, G. F. A mouse model of activating met mutations. Cell Cycle 4, 518–520 (2005).
Gampel, A. et al. VEGF regulates the mobilisation of VEGFR-2/KDR from an intracellular endothelial storage compartment. Blood 108, 2624–2631 (2006).
Kermorgant, S., Zicha, D. & Parker, P. J. Protein kinase C controls microtubule-based traffic but not proteasomal degradation of c-Met. J. Biol. Chem. 278, 28921–28929 (2003).
Kirchhausen, T., Macia, E. & Pelish, H. E. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol. 438, 77–93 (2008).
Hill, T. A. et al. Inhibition of dynamin mediated endocytosis by the dynoles—synthesis and functional activity of a family of indoles. J. Med. Chem. 52, 3762–3773 (2009).
Li, N., Lorinczi, M., Ireton, K. & Elferink, L. A. Specific Grb2-mediated interactions regulate clathrin-dependent endocytosis of the cMet-tyrosine kinase. J. Biol. Chem. 282, 16764–16775 (2007).
Sorkin, A. & von Zastrow, M. Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10, 609–622 (2009).
Shtiegman, K. et al. Defective ubiquitinylation of EGFR mutants of lung cancer confers prolonged signaling. Oncogene 26, 6968–6978 (2007).
Nakaigawa, N., Weirich, G., Schmidt, L. & Zbar, B. Tumorigenesis mediated by MET mutant M1268T is inhibited by dominant-negative Src. Oncogene 19, 2996–3002 (2000).
Ponzetto, C. et al. Specific uncoupling of GRB2 from the Met receptor. Differential effects on transformation and motility. J. Biol. Chem. 271, 14119–14123 (1996).
Egan, S. E. et al. Association of Sos Ras exchange protein with Grb2 is implicated in tyrosine kinase signal transduction and transformation. Nature 363, 45–51 (1993).
Heasman, S. J. & Ridley, A. J. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 9, 690–701 (2008).
Miller, M. et al. Structural basis of oncogenic activation caused by point mutations in the kinase domain of the MET proto-oncogene: modeling studies. Proteins 44, 32–43 (2001).
Xiang, Z., Kreisel, F., Cain, J., Colson, A. & Tomasson, M. H. Neoplasia driven by mutant c-KIT is mediated by intracellular, not plasma membrane, receptor signalling. Mol. Cell Biol. 27, 267–282 (2007).
Runeberg-Roos, P., Virtanen, H. & Saarma, M. RET(MEN 2B) is active in the endoplasmic reticulum before reaching the cell surface. Oncogene 26, 7909–7915 (2007).
Lievens, P. M., Mutinelli, C., Baynes, D. & Liboi, E. The kinase activity of fibroblast growth factor receptor 3 with activation loop mutations affects receptor trafficking and signaling. J. Biol. Chem. 279, 43254–43260 (2004).
Choudhary, C. et al. Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol. Cell 36, 326–339 (2009).
Schmidt-Arras, D. et al. Anchoring of FLT3 in the endoplasmic reticulum alters signaling quality. Blood 113, 3568–3576 (2009).
Scita, G. & Di Fiore, P. P. The endocytic matrix. Nature 463, 464–473.
Muller, P. A. et al. Mutant p53 drives invasion by promoting integrin recycling. Cell 139, 1327–1341 (2009).
Grandal, M. V. et al. EGFRvIII escapes down-regulation due to impaired internalization and sorting to lysosomes. Carcinogenesis 28, 1408–1417 (2007).
Peschard, P. et al. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol. Cell 8, 995–1004 (2001).
Honegger, A. M., Schmidt, A., Ullrich, A. & Schlessinger, J. Separate endocytic pathways of kinase-defective and -active EGF receptor mutants expressed in same cells. J. Cell Biol. 110, 1541–1548 (1990).
Huang, F., Goh, L. K. & Sorkin, A. EGF receptor ubiquitination is not necessary for its internalization. Proc. Natl Acad. Sci. USA 104, 16904–16909 (2007).
Wang, Q., Villeneuve, G. & Wang, Z. Control of epidermal growth factor receptor endocytosis by receptor dimerization, rather than receptor kinase activation. EMBO Rep. 6, 942–948 (2005).
Pellinen, T. & Ivaska, J. Integrin traffic. J. Cell Sci. 119, 3723–3731 (2006).
Assaker, G., Ramel, D., Wculek, S. K., Gonzalez-Gaitan, M. & Emery, G. Spatial restriction of receptor tyrosine kinase activity through a polarized endocytic cycle controls border cell migration. Proc. Natl Acad. Sci. USA 107, 22558–22563 (2010).
Murphy, J. E., Padilla, B. E., Hasdemir, B., Cottrell, G. S. & Bunnett, N. W. Endosomes: a legitimate platform for the signaling train. Proc. Natl Acad. Sci. USA 106, 17615–17622 (2009).
Kong-Beltran, M. et al. Somatic mutations lead to an oncogenic deletion of met in lung cancer. Cancer Res. 66, 283–289 (2006).
Cernuda-Morollon, E., Millan, J., Shipman, M., Marelli-Berg, F. M. & Ridley, A. J. Rac activation by the T-cell receptor inhibits T cell migration. PLoS One 5, e12393 (2010).
Acknowledgements
We thank G. Vande Woude for his gift of the NIH3T3 cell lines expressing murine Met, O. Joffre, V. Carrière, S. Vallath and J. Hulit for their intellectual/technical contributions, A.J. Ridley and S. Heasman for the gift of the GST–PAK-PBD, P. J. Parker and S. Tooze for critically reading the manuscript, J. Camonis, the UK Medical Research Council, and Barts and The London Charitable Foundation.
Author information
Authors and Affiliations
Contributions
C.J. carried out and analysed most experiments using the NIH3T3 cells both in vitro and in vivo, including characterization of the cell lines, western blots, immunofluorescence analyses, Transwell migration, soft-agar assays, flow cytometry analysis, immunoprecipitation and most separation procedures investigating internalization, degradation and recycling. R.B. did most RNAi knockdowns and transfections followed by the subsequent migration assays, soft-agar assays, western blots and immunofluorescence analysis. R.B. carried out transfection and characterization of hM1268T and hM1268T/N1358H mutants in NIH3T3 cells. L.M. carried out Rac1 activation assays following Grb2 RNAi and PHA treatment, established the detection of active Rac and helped with immunofluorescence analyses and analysis of hM1268T and hM1268T/N1358H cells. V.C. designed and developed the hM1268T and hM1268T/N1358H mutants. S.K. conceived the project, designed experiments, interpreted the data and carried out some of the immunofluorescence and confocal microscopy analyses. I.R.H. advised on the design of the in vivo experiments, trained C.J. and carried out inoculations of tumour cells. S.K. and C.J. wrote the manuscript, with additional input from I.R.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1350 kb)
Supplementary Information
Supplementary Table 1 (XLS 30 kb)
Rights and permissions
About this article
Cite this article
Joffre, C., Barrow, R., Ménard, L. et al. A direct role for Met endocytosis in tumorigenesis. Nat Cell Biol 13, 827–837 (2011). https://doi.org/10.1038/ncb2257
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2257
This article is cited by
-
MET exon 14 skipping mutation drives cancer progression and recurrence via activation of SMAD2 signalling
British Journal of Cancer (2024)
-
SAMHD1-induced endosomal FAK signaling promotes human renal clear cell carcinoma metastasis by activating Rac1-mediated lamellipodia protrusion
Experimental & Molecular Medicine (2023)
-
Hypoxic stress disrupts HGF/Met signaling in human trophoblasts: implications for the pathogenesis of preeclampsia
Journal of Biomedical Science (2022)
-
Endosomal LC3C-pathway selectively targets plasma membrane cargo for autophagic degradation
Nature Communications (2022)
-
The role of MET in chemotherapy resistance
Oncogene (2021)