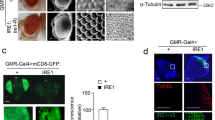
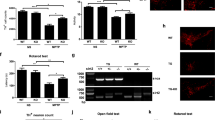
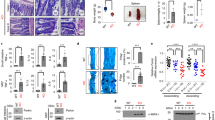
Abstract
Cyclin-dependent kinase 5 (Cdk5) is a serine/threonine kinase that is increasingly implicated in various neurodegenerative diseases. Deregulated Cdk5 activity has been associated with neuronal death, but the underlying mechanisms are not well understood. Here we report an unexpected role for Cdk5 in the regulation of induced autophagy in neurons. We have identified endophilin B1 (EndoB1) as a Cdk5 substrate, and show that Cdk5-mediated phosphorylation of EndoB1 is required for autophagy induction in starved neurons. Furthermore, phosphorylation of EndoB1 facilitates EndoB1 dimerization and recruitment of UVRAG (UV radiation resistance-associated gene). More importantly, Cdk5-mediated phosphorylation of EndoB1 is essential for autophagy induction and neuronal loss in models of Parkinson’s disease. Our findings not only establish Cdk5 as a critical regulator of autophagy induction, but also reveal a role for Cdk5 and EndoB1 in the pathophysiology of Parkinson’s disease through modulating autophagy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
21 April 2011
In the version of this article initially published online and in print, there were some errors in Fig. 3b. These errors have been corrected in the HTML and PDF versions of the article.
References
Dhavan, R. & Tsai, L. H. A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2, 749–759 (2001).
Cheung, Z. H., Fu, A. K. & Ip, N. Y. Synaptic roles of Cdk5: implications in higher cognitive functions and neurodegenerative diseases. Neuron 50, 13–18 (2006).
Cheung, Z. H. & Ip, N. Y. Cdk5: mediator of neuronal death and survival. Neurosci. Lett. 361, 47–51 (2004).
Cheung, Z. H., Gong, K. & Ip, N. Y. Cyclin-dependent kinase 5 supports neuronal survival through phosphorylation of Bcl-2. J. Neurosci. 28, 4872–4877 (2008).
Li, B. S. et al. Cyclin-dependent kinase 5 prevents neuronal apoptosis by negative regulation of c-Jun N-terminal kinase 3. EMBO J. 21, 324–333 (2002).
O’Hare, M. J. et al. Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. J. Neurosci. 25, 8954–8966 (2005).
Smith, P. D. et al. Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proc. Natl Acad. Sci. USA 100, 13650–13655 (2003).
Patrick, G. N. et al. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402, 615–622 (1999).
Smith, P. D. et al. Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J. Neurosci. 26, 440–447 (2006).
Paoletti, P. et al. Dopaminergic and glutamatergic signalling crosstalk in Huntington’s disease neurodegeneration: the role of p25/cyclin-dependent kinase 5. J. Neurosci. 28, 10090–10101 (2008).
Dauer, W. & Przedborski, S. Parkinson’s disease: mechanisms and models. Neuron 39, 889–909 (2003).
Levy, O. A., Malagelada, C. & Greene, L. A. Cell death pathways in Parkinson’s disease: proximal triggers, distal effectors, and final steps. Apoptosis 14, 478–500 (2009).
Qu, D. et al. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson’s disease. Neuron 55, 37–52 (2007).
Huang, E. et al. The role of Cdk5-mediated apurinic/apyrimidinic endonuclease 1 phosphorylation in neuronal death. Nat. Cell Biol. 12, 563–571 (2010).
Rubinsztein, D. C. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature 443, 780–786 (2006).
Maiuri, M. C., Zalckvar, E., Kimchi, A. & Kroemer, G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 8, 741–752 (2007).
Zhu, J. H., Guo, F., Shelburne, J., Watkins, S. & Chu, C. T. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 13, 473–481 (2003).
Anglade, P. et al. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol. Histopathol. 12, 25–31 (1997).
Zhu, J. H. et al. Regulation of autophagy by extracellular signal-regulated protein kinases during 1-methyl-4-phenylpyridinium-induced cell death. Am. J. Pathol. 170, 75–86 (2007).
Stefanis, L., Larsen, K. E., Rideout, H. J., Sulzer, D. & Greene, L. A. Expression of A53T mutant but not wild-type α-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J. Neurosci. 21, 9549–9560 (2001).
Xilouri, M., Vogiatzi, T., Vekrellis, K., Park, D. & Stefanis, L. Aberrant α-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PloS One 4, e5515 (2009).
Cheung, Z. H. & Ip, N. Y. The emerging role of autophagy in Parkinson’s disease. Mol. Brain 2, 29 (2009).
Cuervo, A. M., Stefanis, L., Fredenburg, R., Lansbury, P. T. & Sulzer, D. Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science 305, 1292–1295 (2004).
Vogiatzi, T., Xilouri, M., Vekrellis, K. & Stefanis, L. Wild type α-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J. Biol. Chem. 283, 23542–23556 (2008).
Hara, T. et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 (2006).
Komatsu, M. et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884 (2006).
Ravikumar, B. et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat. Genet. 36, 585–595 (2004).
Levine, B. & Kroemer, G. Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008).
Yang, Q. et al. Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science 323, 124–127 (2009).
Takahashi, Y. et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumourigenesis. Nat. Cell Biol. 9, 1142–1151 (2007).
Modregger, J., Schmidt, A. A., Ritter, B., Huttner, W. B. & Plomann, M. Characterization of endophilin B1b, a brain-specific membrane-associated lysophosphatidic acid acyl transferase with properties distinct from endophilin A1. J. Biol. Chem. 278, 4160–4167 (2003).
Farsad, K. et al. Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J. Cell Biol. 155, 193–200 (2001).
Cuddeback, S. M. et al. Molecular cloning and characterization of Bif-1. A novel Src homology 3 domain-containing protein that associates with Bax. J. Biol. Chem. 276, 20559–20565 (2001).
Gallop, J. L. et al. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 25, 2898–2910 (2006).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4, 151–175 (2008).
Mizushima, N., Yoshimori, T. & Levine, B. Methods in mammalian autophagy research. Cell 140, 313–326 (2010).
Huttner, W. B. & Schmidt, A. A. Membrane curvature: a case of endofeelin. Trends Cell Biol. 12, 155–158 (2002).
Liang, C. et al. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat. Cell Biol. 10, 776–787 (2008).
Liang, C. et al. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat. Cell Biol. 8, 688–699 (2006).
Martin, L. J. et al. Parkinson’s disease α-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 26, 41–50 (2006).
Giasson, B. I. et al. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 34, 521–533 (2002).
Mizushima, N., Levine, B., Cuervo, A. M. & Klionsky, D. J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008).
Schmelzle, T. & Hall, M. N. TOR, a central controller of cell growth. Cell 103, 253–262 (2000).
Bredesen, D. E., Rao, R. V. & Mehlen, P. Cell death in the nervous system. Nature 443, 796–802 (2006).
Wan, J. et al. Endophilin B1 as a novel regulator of nerve growth factor/ TrkA trafficking and neurite outgrowth. J. Neurosci. 28, 9002–9012 (2008).
McPhee, C. K., Logan, M. A., Freeman, M. R. & Baehrecke, E. H. Activation of autophagy during cell death requires the engulfment receptor Draper. Nature 465, 1093–1096 (2010).
Shibata, M. et al. Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J. Biol. Chem. 281, 14474–14485 (2006).
Fu, W. Y. et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat. Neurosci. 10, 67–76 (2007).
Cheung, Z. H., Chin, W. H., Chen, Y., Ng, Y. P. & Ip, N. Y. Cdk5 is involved in BDNF-stimulated dendritic growth in hippocampal neurons. PLoS Biol. 5, e63 (2007).
Lee, J. A. & Gao, F. B. Inhibition of autophagy induction delays neuronal cell loss caused by dysfunctional ESCRT-III in frontotemporal dementia. J. Neurosci. 29, 8506–8511 (2009).
Cheng, K. et al. Pctaire1 interacts with p35 and is a novel substrate for Cdk5/p35. J. Biol. Chem. 277, 31988–31993 (2002).
Lai, K. O. et al. Identification of the Jak/Stat proteins as novel downstream targets of EphA4 signalling in muscle: implications in the regulation of acetylcholinesterase expression. J. Biol. Chem. 279, 13383–13392 (2004).
Jin, W. et al. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J. Neurosci. 26, 2380–2390 (2006).
Acknowledgements
We are grateful to A. B. Kulkarni (National Institutes of Health, USA) and T. Curran (St Jude Children’s Research Hospital, USA) for the Cdk5-knockout mice. p35-knockout mice were provided by L. H. Tsai (Massachusetts Institute of Technology, USA). We thank T. Yoshimori (Osaka University, Japan) for the mRFP–LC3 construct, V. M. Y. Lee (University of Pennsylvania School of Medicine, USA) for the SNL-1 antibody and wild-type α-synuclein and α-synucleinA53T constructs and J. Yuan (Harvard Medical School) for the pcDNA3-Beclin 1 construct. We thank J. Li, G. Ke, W. Y. Fu, K. Cheng, J. Wan, Y. P. Ng, V. Lee and A. Lai for technical assistance, and members of the Ip laboratory for discussions. This work was supported in part by the Research Grants Council of Hong Kong (HKUST 661007, 661109, 660309, 660210, 1/06C and 6/CRF/08), the Area of Excellence Scheme of the University Grants Committee (AoE/B-15/01) and the Hong Kong Jockey Club. N.Y.I. and Z.H.C. were recipients of the Croucher Foundation Senior Research Fellowship and Croucher Foundation Fellowship, respectively.
Author information
Authors and Affiliations
Contributions
A.S.L.W., R.H.K.L., A.Y.C. and P.K.Y. carried out the experiments. A.S.L.W., R.H.K.L., A.Y.C., P.K.Y., Z.H.C. and N.Y.I. planned the studies, designed the experiments and interpreted the results. S.K.C., Z.H.C. and N.Y.I. provided reagents and resources. A.S.L.W., Z.H.C. and N.Y.I. wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1530 kb)
Rights and permissions
About this article
Cite this article
Wong, A., Lee, R., Cheung, A. et al. Cdk5-mediated phosphorylation of endophilin B1 is required for induced autophagy in models of Parkinson's disease. Nat Cell Biol 13, 568–579 (2011). https://doi.org/10.1038/ncb2217
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2217
This article is cited by
-
SH3GLB1-related autophagy mediates mitochondrial metabolism to acquire resistance against temozolomide in glioblastoma
Journal of Experimental & Clinical Cancer Research (2022)
-
Endophilin A2 regulates calcium-activated chloride channel activity via selective autophagy-mediated TMEM16A degradation
Acta Pharmacologica Sinica (2020)
-
Quantitative Phosphoproteomic Analysis in Alpha-Synuclein Transgenic Mice Reveals the Involvement of Aberrant p25/Cdk5 Signaling in Early-stage Parkinson’s Disease
Cellular and Molecular Neurobiology (2020)
-
Silymarin Protects Against Impaired Autophagy Associated with 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Parkinsonism
Journal of Molecular Neuroscience (2020)
-
Roscovitine effectively enhances antitumor activity of temozolomide in vitro and in vivo mediated by increased autophagy and Caspase-3 dependent apoptosis
Scientific Reports (2019)