Abstract
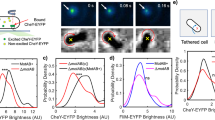
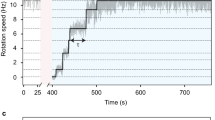
Ciliates and flagellates temporarily swim backwards on collision by generating a mechanoreceptor potential. Although this potential has been shown to be associated with cilia in Paramecium, the molecular entity of the mechanoreceptor has remained unknown. Here we show that Chlamydomonas cells express TRP11, a member of the TRP (transient receptor potential) subfamily V, in the proximal region of the flagella, and that suppression of TRP11 expression results in loss of the avoiding reaction. The results indicate that Chlamydomonas flagella exhibit mechanosensitivity, despite constant motility, by localizing the mechanoreceptor in the proximal region, where active bending is restricted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Köttgen, M. TRPP2 and autosomal dominant polycystic kidney disease. Biochim. Biophys. Acta 1772, 836–850 (2007).
Naitoh, Y. & Eckert, R. Ionic mechanisms controlling behavioral responses of paramecium to mechanical stimulation. Science 164, 963–965 (1969).
Ogura, A. & Takahashi, K. Artificial deciliation causes loss of calcium-dependent responses in Paramecium. Nature 264, 170–172 (1976).
Damann, N., Voets, T. & Nilius, B. TRPs in our senses. Curr. Biol. 18, R880–R889 (2008).
Fujiu, K., Nakayama, Y., Yanagisawa, A., Sokabe, M. & Yoshimura, K. Chlamydomonas CAV2 encodes a voltage-dependent calcium channel required for the flagellar waveform conversion. Curr. Biol. 19, 133–139 (2009).
Merchant, S. S. et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318, 245–250 (2007).
Huang, K. et al. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J. Cell Biol 179, 501–514 (2007).
Yoshimura, K. A novel type of mechanoreception by the flagella of Chlamydomonas. J. Exp. Biol. 199, 295–302 (1996).
Remillard, S. P. & Witman, G. B. Synthesis, transport, and utilization of specific flagellar proteins during flagellar regeneration in Chlamydomonas. J. Cell Biol 93, 615–631 (1982).
Yagi, T., Uematsu, K., Liu, Z. & Kamiya, R. Identification of dyneins that localize exclusively to the proximal portion of Chlamydomonas flagella. J. Cell Sci. 122, 1306–1314 (2009).
Witman, G. B., Plummer, J. & Sander, G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. Structure, composition, and function of specific axonemal components. J. Cell Biol. 76, 729–747 (1978).
Qin, H. et al. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr. Biol. 15, 1695–1699 (2005).
Minke, B. TRP channels and Ca2+ signaling. Cell Calcium 40, 261–275 (2006).
Colbert, H. A, Smith, T. L. & Bargmann, C. I. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 17, 8259–8269 (1997).
Tobin, D. et al. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35, 307–318 (2002).
Kim, J. et al. A TRPV family ion channel required for hearing in Drosophila. Nature 424, 81–84 (2003).
Lorenzo, I. M. et al. TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc. Natl Acad. Sci. USA 105, 12611–12616 (2008).
Huang, B., Rifkin, M. R. & Luck, D. J. Temperature-sensitive mutations affecting flagellar assembly and function in Chlamydomonas reinhardtii. J. Cell Biol. 72, 67–85 (1977).
Gorman, D. S. & Levine, R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc. Natl Acad. Sci. USA 54, 1665–1669 (1965).
Sineshchekov, O. A., Jung, K. H. & Spudich, J. L. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc. Natl Acad. Sci. USA 99, 8689–8694 (2002).
Acknowledgements
This work was supported by a grant from the Japan Science and Technology Agency and Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan under grant numbers 10J02008 (to Y.N.), 21026009, 21370017 (to H.I.), 21247021, 16GS0308 (to M.S.) and 16370034 (to K.Y.).
Author information
Authors and Affiliations
Contributions
K.F., Y. N. and K.Y. carried out the experiments; K.F. and K.Y. designed the study; M.S. and H.I. gave conceptual advice; K.Y. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 374 kb)
Rights and permissions
About this article
Cite this article
Fujiu, K., Nakayama, Y., Iida, H. et al. Mechanoreception in motile flagella of Chlamydomonas. Nat Cell Biol 13, 630–632 (2011). https://doi.org/10.1038/ncb2214
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2214
This article is cited by
-
What is it like to be a choanoflagellate? Sensation, processing and behavior in the closest unicellular relatives of animals
Animal Cognition (2023)
-
High hydrostatic pressure induces vigorous flagellar beating in Chlamydomonas non-motile mutants lacking the central apparatus
Scientific Reports (2020)
-
Identification and Characterization of a Transient Receptor Potential Ion Channel (TRP2) Involved in Acclimation to Low CO2 Conditions in Chlamydomonas reinhardtii
Plant Molecular Biology Reporter (2020)
-
Structure of the thermo-sensitive TRP channel TRP1 from the alga Chlamydomonas reinhardtii
Nature Communications (2019)
-
Cell wall integrity signaling regulates cell wall-related gene expression in Chlamydomonas reinhardtii
Scientific Reports (2019)