Abstract
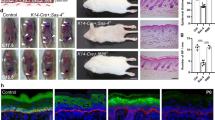
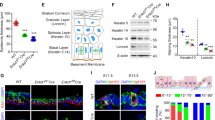
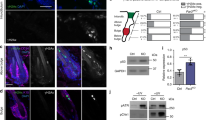
During development, a polarized epidermal sheet undergoes stratification and differentiation to produce the skin barrier. Through mechanisms that are poorly understood, the process involves actin dynamics, spindle reorientation and Notch signalling. To elucidate how epidermal embryogenesis is governed, we conditionally targeted serum response factor (Srf), a transcription factor that is essential for epidermal differentiation. Unexpectedly, previously ascribed causative defects are not responsible for profoundly perturbed embryonic epidermis. Seeking the mechanism for this, we identified actins and their regulators that were downregulated after ablation. Without Srf, cells exhibit a diminished cortical network and in mitosis, they fail to round up, features we recapitulate with low-dose actin inhibitors in vivo and shRNA-knockdown in vitro. Altered concomitantly are phosphorylated ERM and cortical myosin-IIA, shown in vitro to establish a rigid cortical actomyosin network and elicit critical shape changes. We provide a link between these features and Srf loss, and we show that the process is physiologically relevant in skin, as reflected by defects in spindle orientation, asymmetric cell divisions, stratification and differentiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Blanpain, C. & Fuchs, E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 10, 207–217 (2009).
Perez-Moreno, M., Jamora, C. & Fuchs, E. Sticky business: orchestrating cellular signals at adherens junctions. Cell 112, 535–548 (2003).
Lechler, T. & Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280 (2005).
Kunda, P. & Baum, B. The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol. 19, 174–179 (2009).
Thery, M. & Bornens, M. Cell shape and cell division. Curr. Opin. Cell Biol. 18, 648–657 (2006).
Carreno, S. et al. Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells. J. Cell Biol. 180, 739–746 (2008).
Kunda, P., Pelling, A. E., Liu, T. & Baum, B. Moesin controls cortical rigidity, cell rounding, and spindle morphogenesis during mitosis. Curr. Biol. 18, 91–101 (2008).
Thery, M. et al. The extracellular matrix guides the orientation of the cell division axis. Nat. Cell Biol. 7, 947–953 (2005).
Kaji, N., Muramoto, A. & Mizuno, K. LIM kinase-mediated cofilin phosphorylation during mitosis is required for precise spindle positioning. J. Biol. Chem. 283, 4983–4992 (2008).
Cramer, L. P. & Mitchison, T. J. Investigation of the mechanism of retraction of the cell margin and rearward flow of nodules during mitotic cell rounding. Mol. Biol. Cell 8, 109–119 (1997).
Cuenca, A. A., Schetter, A., Aceto, D., Kemphues, K. & Seydoux, G. Polarization of the C. elegans zygote proceeds via distinct establishment and maintenance phases. Development 130, 1255–1265 (2003).
Severson, A. F. & Bowerman, B. Myosin and the PAR proteins polarize microfilament-dependent forces that shape and position mitotic spindles in Caenorhabditis elegans. J. Cell Biol. 161, 21–26 (2003).
Munro, E., Nance, J. & Priess, J. R. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior–posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413–424 (2004).
Grill, S. W., Gonczy, P., Stelzer, E. H. & Hyman, A. A. Polarity controls forces governing asymmetric spindle positioning in the Caenorhabditis elegans embryo. Nature 409, 630–633 (2001).
Larson, S. M. et al. Cortical mechanics and meiosis II completion in mammalian oocytes are mediated by myosin-II and Ezrin–Radixin–Moesin (ERM) proteins. Mol. Biol. Cell 15, 3182–3192 (2010).
Verdoni, A. M., Ikeda, S. & Ikeda, A. Serum response factor is essential for the proper development of skin epithelium. Mamm. Genome 21, 64–76 (2010).
Koegel, H. et al. Loss of serum response factor in keratinocytes results in hyperproliferative skin disease in mice. J. Clin. Invest. 119, 899–910 (2009).
Connelly, J. T. et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat. Cell Biol. 12, 711–718 (2010).
Posern, G. & Treisman, R. Actin’ together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 16, 588–596 (2006).
Miano, J. M., Long, X. & Fujiwara, K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am. J. Physiol. Cell Physiol. 292, C70–C81 (2007).
Miano, J. M. et al. Restricted inactivation of serum response factor to the cardiovascular system. Proc. Natl Acad. Sci. USA 101, 17132–17137 (2004).
Li, S. et al. Requirement for serum response factor for skeletal muscle growth and maturation revealed by tissue-specific gene deletion in mice. Proc. Natl Acad. Sci. USA 102, 1082–1087 (2005).
Alberti, S. et al. Neuronal migration in the murine rostral migratory stream requires serum response factor. Proc. Natl Acad. Sci. USA 102, 6148–6153 (2005).
Franco, C. A. et al. Serum response factor is required for sprouting angiogenesis and vascular integrity. Dev. Cell 15, 448–461 (2008).
Medjkane, S., Perez-Sanchez, C., Gaggioli, C., Sahai, E. & Treisman, R. Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nat. Cell Biol. 11, 257–268 (2009).
Vasioukhin, V., Degenstein, L., Wise, B. & Fuchs, E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl Acad. Sci. USA 96, 8551–8556 (1999).
Fuchs, E. & Green, H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell 19, 1033–1042 (1980).
Coulombe, P. A., Kerns, M. L. & Fuchs, E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J. Clin. Invest. 119, 1784–1793 (2009).
Watt, F. M., Estrach, S. & Ambler, C. A. Epidermal Notch signalling: differentiation, cancer and adhesion. Curr. Opin. Cell Biol. 20, 171–179 (2008).
Rangarajan, A. et al. Notch signalling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 20, 3427–3436 (2001).
Demehri, S. & Kopan, R. Notch signaling in bulge stem cells is not required for selection of hair follicle fate. Development 136, 891–896 (2009).
Blanpain, C., Lowry, W. E., Pasolli, H. A. & Fuchs, E. Canonical Notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 20, 3022–3035 (2006).
Moriyama, M. et al. Multiple roles of Notch signaling in the regulation of epidermal development. Dev. Cell 14, 594–604 (2008).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Sun, Q. et al. Defining the mammalian CArGome. Genome Res. 16, 197–207 (2006).
Huang, Q. Q. et al. Role of H2-calponin in regulating macrophage motility and phagocytosis. J. Biol. Chem. 283, 25887–25899 (2008).
Hossain, M. M., Smith, P. G., Wu, K. & Jin, J. P. Cytoskeletal tension regulates both expression and degradation of h2-calponin in lung alveolar cells. Biochemistry 45, 15670–15683 (2006).
Hossain, M. M., Crish, J. F., Eckert, R. L., Lin, J. J. & Jin, J. P. h2-Calponin is regulated by mechanical tension and modifies the function of actin cytoskeleton. J. Biol. Chem. 280, 42442–42453 (2005).
Feng, Y. & Walsh, C. A. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat. Cell Biol. 6, 1034–1038 (2004).
Fujibuchi, T. et al. AIP1/WDR1 supports mitotic cell rounding. Biochem. Biophys. Res. Commun. 327, 268–275 (2005).
Fang, M. et al. Evidence of EGR1 as a differentially expressed gene among proliferative skin diseases. Genomic. Med. 1, 75–85 (2007).
Kumbrink, J., Kirsch, K. H. & Johnson, J. P. EGR1, EGR2, and EGR3 activate the expression of their coregulator NAB2 establishing a negative feedback loop in cells of neuroectodermal and epithelial origin. J. Cell Biochem. 111, 207–217 (2010).
Kubosaki, A. et al. Genome-wide investigation of in vivo EGR-1 binding sites in monocytic differentiation. Genome Biol. 10, R41 (2009).
Thyss, R. et al. NF-κB/Egr-1/Gadd45 are sequentially activated upon UVB irradiation to mediate epidermal cell death. EMBO J. 24, 128–137 (2005).
Thery, M. & Bornens, M. Get round and stiff for mitosis. Hfsp J. 2, 65–71 (2008).
Morton, W. M., Ayscough, K. R. & McLaughlin, P. J. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat. Cell Biol. 2, 376–378 (2000).
Ayscough, K. R. et al. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137, 399–416 (1997).
Siller, K. H. & Doe, C. Q. Spindle orientation during asymmetric cell division. Nat. Cell Biol. 11, 365–374 (2009).
Neumuller, R. A. & Knoblich, J. A. Dividing cellular asymmetry: asymmetric cell division and its implications for stem cells and cancer. Genes Dev. 23, 2675–2699 (2009).
Zheng, Z. et al. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J. Cell Biol. 19, 275–288 (2010).
Morin, X., Jaouen, F. & Durbec, P. Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat. Neurosci. 10, 1440–1448 (2007).
Konno, D. et al. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nat. Cell Biol. 10, 93–101 (2008).
Bubb, M. R., Senderowicz, A. M., Sausville, E. A., Duncan, K. L. & Korn, E. D. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 269, 14869–14871 (1994).
Straight, A. F. et al. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science 299, 1743–1747 (2003).
Effler, J. C. et al. Mitosis-specific mechanosensing and contractile-protein redistribution control cell shape. Curr. Biol. 16, 1962–1967 (2006).
Cabernard, C., Prehoda, K. E. & Doe, C. Q. A spindle-independent cleavage furrow positioning pathway. Nature 467, 91–94 (2010).
Knoblich, J. A. Mechanisms of asymmetric stem cell division. Cell 132, 583–597 (2008).
Ou, G. et al. Polarized myosin produces unequal-size daughters during asymmetric cell division. Science 330, 677–680 (2010).
Li, R. & Gundersen, G. G. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nat. Rev. Mol. Cell Biol. 9, 860–873 (2008).
Chai, J. & Tarnawski, A. S. Serum response factor: discovery, biochemistry, biological roles and implications for tissue injury healing. J. Physiol. Pharmacol. 53, 147–157 (2002).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Vasioukhin, V. & Fuchs, E. Actin dynamics and cell–cell adhesion in epithelia. Curr. Opin. Cell Biol. 13, 76–84 (2001).
Beronja, S., Livshits, G., Williams, S. & Fuchs, E. Rapid functional dissection of genetic networks via tissue-specific transduction and RNAi in mouse embryos. Nat. Med. 16, 821–827 (2010).
Acknowledgements
We thank the RU Facilities for Bioimaging (A. North) and FACS (S. Mazel) staff for their technical support; we thank N. Stokes for her assistance in the mouse facility and the Comparative Biology Center (CBC) staff for their help in veterinary care and health of our mice. We are grateful to E. Ezratty, S. Beronja, A. R. Folgueras, M. Nikolova and other members of the Fuchs laboratory for helpful discussions, critical reading of the manuscript and experimental assistance. C.L. is a Tri-Institutional Starr Stem Cell Scholars Posdoctoral Fellow. S.E.W. is an American Cancer Society Postdoctoral Fellow. Work in the Fuchs laboratory was supported by a grant from the National Institutes of Health (E.F. R01AR27883).
Author information
Authors and Affiliations
Contributions
C.L. and H.A.P. carried out the experiments and analysed the raw data. S.E.W. contributed reagents and experimental suggestions. C.L. and E.F. wrote the manuscript. E.F. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2505 kb)
Rights and permissions
About this article
Cite this article
Luxenburg, C., Amalia Pasolli, H., Williams, S. et al. Developmental roles for Srf, cortical cytoskeleton and cell shape in epidermal spindle orientation. Nat Cell Biol 13, 203–214 (2011). https://doi.org/10.1038/ncb2163
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2163
This article is cited by
-
RNAi-directed knockdown in the cnidarian fish blood parasite Sphaerospora molnari
Scientific Reports (2024)
-
Profilin 1 deficiency drives mitotic defects and reduces genome stability
Communications Biology (2023)
-
Anillin governs mitotic rounding during early epidermal development
BMC Biology (2022)
-
High proliferation and delamination during skin epidermal stratification
Nature Communications (2021)
-
Targeting the actin/tropomyosin cytoskeleton in epithelial ovarian cancer reveals multiple mechanisms of synergy with anti-microtubule agents
British Journal of Cancer (2021)