Abstract
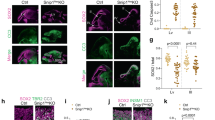
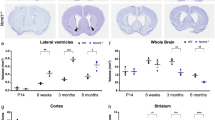
To better understand the mechanisms that regulate stem cell identity and function, we sought to identify genes that are preferentially expressed by stem cells and critical for their function in multiple tissues. Prdm16 is a transcription factor that regulates leukaemogenesis1, palatogenesis2 and brown-fat development3,4,5, but which was not known to be required for stem cell function. We demonstrate that Prdm16 is preferentially expressed by stem cells throughout the nervous and haematopoietic systems and is required for their maintenance. In the haematopoietic and nervous systems, Prdm16 deficiency led to changes in the levels of reactive oxygen species (ROS), depletion of stem cells, increased cell death and altered cell-cycle distribution. In neural stem/progenitor cells, Prdm16 binds to the Hgf promoter, and Hgf expression declined in the absence of Prdm16. Addition of recombinant HGF to Prdm16-deficient neural stem cells in cell culture reduced the depletion of these cells and partially rescued the increase in ROS levels. Administration of the anti-oxidant, N-acetyl-cysteine, to Prdm16-deficient mice partially rescued defects in neural stem/progenitor cell function and neural development. Prdm16 therefore promotes stem cell maintenance in multiple tissues, partly by modulating oxidative stress.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Morishita, K. Leukemogenesis of the EVI1/MEL1 gene family. Int. J. Hematol. 85, 279–286 (2007).
Bjork, B. C., Turbe-Doan, A., Prysak, M., Herron, B. J. & Beier, D. R. Prdm16 is required for normal palatogenesis in mice. Hum. Mol. Genet. 19, 774–789 (2010).
Seale, P. et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008).
Seale, P. et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6, 38–54 (2007).
Murholm, M. et al. Dynamic regulation of genes involved in mitochondrial DNA replication and transcription during mouse brown fat cell differentiation and recruitment. PloS one 4, e8458 (2009).
He, S., Nakada, D. & Morrison, S. J. Mechanisms of stem cell self-renewal. Annu. Rev. Cell Dev. Biol. 25, 377–406 (2009).
Miyamoto, K. et al. FoxO3a is essential for maintenance of the hematopoietic stem cell pool. Cell stem cell 1, 101–112 (2007).
Paik, J. H. et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell stem cell 5, 540–553 (2009).
Renault, V. M. et al. FoxO3 regulates neural stem cell homeostasis. Cell stem cell 5, 527–539 (2009).
Tothova, Z. et al. FoxOs are critical mediators of haematopoietic stem cell resistance to physiologic oxidative stress. Cell 128, 325–339 (2007).
Valk-Lingbeek, M. E., Bruggeman, S. W. & van Lohuizen, M. Stem cells and cancer; the polycomb connection. Cell 118, 409–418 (2004).
Rossi, D. J., Jamieson, C. H. & Weissman, I. L. Stems cells and the pathways to aging and cancer. Cell 132, 681–696 (2008).
Goyama, S. et al. Evi-1 is a critical regulator for hematopoietic stem cells and transformed leukemic cells. Cell stem cell 3, 207–220 (2008).
Kim, I., Saunders, T. L. & Morrison, S. J. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 130, 470–483 (2007).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Jaks, V. et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 40, 1291–1299 (2008).
Du, Y., Jenkins, N. A. & Copeland, N. G. Insertional mutagenesis identifies genes that promote the immortalization of primary bone marrow progenitor cells. Blood 106, 3932–3939 (2005).
Deneault, E. et al. A functional screen to identify novel effectors of hematopoietic stem cell activity. Cell 137, 369–379 (2009).
Kiel, M. J., Yilmaz, O. H., Iwashita, T., Terhorst, C. & Morrison, S. J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Kiel, M. J., Yilmaz, O. H. & Morrison, S. J. CD150- cells are transiently reconstituting multipotent progenitors with little or no stem cell activity. Blood 111, 4413–4414 (2008).
Ren, T., Zhang, J., Plachez, C., Mori, S. & Richards, L. J. Diffusion tensor magnetic resonance imaging and tract-tracing analysis of Probst bundle structure in Netrin1- and DCC-deficient mice. J. Neurosci. 27, 10345–10349 (2007).
West, A. K., Hidalgo, J., Eddins, D., Levin, E. D. & Aschner, M. Metallothionein in the central nervous system: roles in protection, regeneration and cognition. Neurotoxicology 29, 489–503 (2008).
Ozaki, M., Haga, S., Zhang, H. Q., Irani, K. & Suzuki, S. Inhibition of hypoxia/reoxygenation-induced oxidative stress in HGF-stimulated antiapoptotic signaling: role of PI3-K and Akt kinase upon rac1. Cell Death Differ. 10, 508–515 (2003).
Yoon, Y. S., Lee, J. H., Hwang, S. C., Choi, K. S. & Yoon, G. TGF β1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene 24, 1895–1903 (2005).
Balaban, R. S., Nemoto, S. & Finkel, T. Mitochondria, oxidants, and aging. Cell 120, 483–495 (2005).
Liu, J. et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 459, 387–392 (2009).
Nishino, J., Kim, I., Chada, K. & Morrison, S. J. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf expression. Cell 135, 227–239 (2008).
Irizarry, R. A. et al. Exploration, normalization and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Shamir, R. et al. EXPANDER--an integrative program suite for microarray data analysis. BMC Bioinformatics 6, 232 (2005).
Acknowledgements
This work was supported by the Howard Hughes Medical Institute and by the National Institutes of Neurological Disease and Stroke (NS40750). S.C. was supported by a career development award from the Leukemia and Lymphoma Society. B.P.L. was supported by an American Heart Association Postdoctoral Fellowship (0725726Z) and an Irvington Institute-Cancer Research Institute/Edmond J. Safra Memorial Fellowship. Flow-cytometry work was partially supported by the UM-Comprehensive Cancer, NIH CA46592. We thank M. White and D. Adams for flow-cytometry assistance, G. Wendt for technical assistance, and E. Smith (Hybridoma Core Facility) for antibody production, partially supported through the Rheumatic Core Disease Center (P30 AR48310).
Author information
Authors and Affiliations
Contributions
S.C. and B.P.L. characterized Prdm16 expression and function with assistance from M.L.S. S.C., B.P.L., and S.J.M. designed experiments, interpreted results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–4 (PDF 804 kb)
Rights and permissions
About this article
Cite this article
Chuikov, S., Levi, B., Smith, M. et al. Prdm16 promotes stem cell maintenance in multiple tissues, partly by regulating oxidative stress. Nat Cell Biol 12, 999–1006 (2010). https://doi.org/10.1038/ncb2101
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2101
This article is cited by
-
Redox-dependent Igfbp2 signaling controls Brca1 DNA damage response to govern neural stem cell fate
Nature Communications (2023)
-
Current Update on Categorization of Migraine Subtypes on the Basis of Genetic Variation: a Systematic Review
Molecular Neurobiology (2023)
-
Doxorubicin induces cardiotoxicity in a pluripotent stem cell model of aggressive B cell lymphoma cancer patients
Basic Research in Cardiology (2022)
-
Inhibition of nuclear factor (erythroid-derived 2)-like 2 promotes hepatic progenitor cell activation and differentiation
npj Regenerative Medicine (2021)
-
Cellular stress signaling activates type-I IFN response through FOXO3-regulated lamin posttranslational modification
Nature Communications (2021)