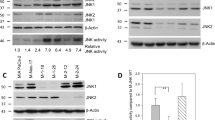
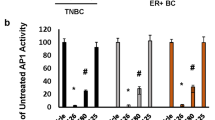
Abstract
The AP-1 transcription factor c-Jun is essential for cellular proliferation in many cell types, but the molecular link between growth factors and c-Jun activation has been enigmatic. In this study we identify a previously uncharacterized RING-domain-containing protein, RACO-1 (RING domain AP-1 co-activator-1), as a c-Jun co-activator that is regulated by growth factor signalling. RACO-1 interacted with c-Jun independently of amino-terminal phosphorylation, and was both necessary and sufficient for c-Jun/AP-1 activation. Growth factor-mediated stimulation of AP-1 was attributable to MEK/ERK-dependent stabilization of RACO-1 protein. Stimulation of the MEK/ERK pathway strongly promoted Lys 63-linked ubiquitylation of RACO-1, which antagonized Lys 48-linked degradative auto-ubiquitylation of the same Lys residues. RACO-1 depletion reduced cellular proliferation and decreased expression of several growth-associated AP-1 target genes, such as cdc2, cyclinD1 and hb-egf. Moreover, transgenic overexpression of RACO-1 augmented intestinal tumour formation triggered by aberrant Wnt signalling and cooperated with oncogenic Ras in colonic hyperproliferation. Thus RACO-1 is a co-activator that links c-Jun to growth factor signalling and is essential for AP-1 function in proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Shaulian, E. & Karin, M. AP-1 in cell proliferation and survival. Oncogene 20, 2390–2400 (2001).
Eferl, R. & Wagner, E. F. AP-1: a double-edged sword in tumorigenesis. Nature Rev. Cancer 3, 859–868 (2003).
Mechta-Grigoriou, F., Gerald, D. & Yaniv, M. The mammalian Jun proteins: redundancy and specificity. Oncogene 20, 2378–2389 (2001).
Davis, R. J. Signal transduction by the JNK group of MAP kinases. Cell 103, 239–252 (2000).
Angel, P., Hattori, K., Smeal, T. & Karin, M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP1. Cell 55, 875–885 (1988).
Chen, Z. et al. MAP kinases. Chem. Rev. 101, 2449–2476 (2001).
Pearson, G. et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22, 153–183 (2001).
Johnson, R. S., van Lingen, B., Papaioannou, V. E. & Spiegelmann, B. M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 7, 1309–1317 (1993).
Schreiber, M. et al. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 13, 607–619 (1999).
Behrens, A., Sibilia, M. & Wagner, E. F. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nature Genet. 21, 326–329 (1999).
Eferl, R. et al. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell 112, 181–192 (2003).
Hilberg, F., Aguzzi, A., Howells, N. & Wagner, E. F. c-jun is essential for normal mouse development and hepatogenesis. Nature 365, 179–181 (1993).
Behrens, A. et al. Impaired intervertebral disc formation in the absence of Jun. Development 130, 103–109 (2003).
Eferl, R. et al. Functions of c-Jun in liver and heart development. J. Cell Biol. 145, 1049–1061 (1999).
Behrens, A., Jochum, W., Sibilia, M. & Wagner, E. F. Oncogenic transformation by ras and fos is mediated by c-Jun N-terminal phosphorylation. Oncogene 19, 2657–2663 (2000).
Lee, W., Mitchell, P. & Tjian, R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell 49, 741–752 (1987).
Angel, P. et al. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell 49, 729–739 (1987).
Morton, S., Davis, R. J., McLaren, A. & Cohen, P. A reinvestigation of the multisite phosphorylation of the transcription factor c-Jun. EMBO J. 22, 3876–3886 (2003).
Nateri, A. S., Riera-Sans, L., Da Costa, C. & Behrens, A. The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science 303, 1374–1378 (2004).
Ardley, H. C. & Robinson, P. A. E3 ubiquitin ligases. Essays Biochem. 41, 15–30 (2005).
Fang, D. et al. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nature Immunol. 3, 281–287 (2002).
Gao, M. et al. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science 306, 271–275 (2004).
Wei, W., Jin, J., Schlisio, S., Harper, J. W. & Kaelin, W. G., Jr. The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell 8, 25–33 (2005).
Wertz, I. E et al. Human De-etiolated-1 regulates c-Jun by assembling a CUL4A ubiquitin ligase. Science 303, 1371–1374 (2004).
De Cesare, D. et al. Heterodimerization of c-Jun with ATF-2 and c-Fos is required for positive and negative regulation of the human urokinase enhancer. Oncogene 11, 365–376 (1995).
Nateri, A. S., Spencer-Dene, B. & Behrens, A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature 437, 281–285 (2005).
Wada, T. et al. MKK7 couples stress signalling to G2/M cell-cycle progression and cellular senescence. Nature Cell Biol. 6, 215–226 (2004).
Yujiri, T., Sather, S., Fanger, G. R. & Johnson, G. L. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science 282, 1911–1914 (1998).
Mansour, S. J., Candia, J. M., Gloor, K. K. & Ahn, N. G. Constitutively active mitogen-activated protein kinase kinase 1 (MAPKK1) and MAPKK2 mediate similar transcriptional and morphological responses. Cell Growth Differ. 7, 243–250 (1996).
Wisdom, R., Johnson, R. S. & Moore, C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. Embo J. 18, 188–197 (1999).
Nyabi, O. et al. Efficient mouse transgenesis using Gateway-compatible ROSA26 locus targeting vectors and F1 hybrid ES cells. Nucleic Acids Res. 37, e55 (2009).
el Marjou, F. et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 (2004).
D'Abaco, G. M., Whitehead, R. H. & Burgess, A. W. Synergy between Apc min and an activated ras mutation is sufficient to induce colon carcinomas. Mol. Cell Biol. 16, 884–891 (1996).
Janssen, K. P. et al. APC and oncogenic KRAS are synergistic in enhancing Wnt signaling in intestinal tumor formation and progression. Gastroenterology 131, 1096–1109 (2006).
Tuveson, D. A. et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell 5, 375–387 (2004).
Bohmann, D. et al. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science 238, 1386–1392 (1987).
Treisman, R. Journey to the surface of the cell: Fos regulation and the SRE. EMBO J. 14, 4905–4913 (1995).
Hu, E. et al. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 13, 3094–3103 (1994).
Shaulian, E. et al. The mammalian UV response: c-Jun induction is required for exit from p53-imposed growth arrest. Cell 103, 897–907 (2000).
Johnson, R., Spiegelman, B., Hanahan, D. & Wisdom, R. Cellular transformation and malignancy induced by ras require c-jun. Mol. Cell Biol. 16, 4504–4511 (1996).
Minden, A., Lin, A., Claret, F. X., Abo, A. & Karin, M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81, 1147–1157 (1995).
de Ruiter, N. D., Wolthuis, R. M., van Dam, H., Burgering, B. M. & Bos, J. L. Ras-dependent regulation of c-Jun phosphorylation is mediated by the Ral guanine nucleotide exchange factor-Ral pathway. Mol. Cell Biol. 20, 8480–8488 (2000).
Kennedy, N. J. et al. Suppression of Ras-stimulated transformation by the JNK signal transduction pathway. Genes Dev. 17, 629–637 (2003).
Alberts, A. S., Geneste, O. & Treisman, R. Activation of SRF-regulated chromosomal templates by Rho-family GTPases requires a signal that also induces H4 hyperacetylation. Cell 92, 475–487 (1998).
Campanero, M. R. & Flemington, E. K. Regulation of E2F through ubiquitin-proteasome-dependent degradation: stabilization by the pRB tumor suppressor protein. Proc. Natl Acad. Sci. USA 94, 2221–2226 (1997).
George, S. H. et al. Developmental and adult phenotyping directly from mutant embryonic stem cells. Proc. Natl Acad. Sci. USA 104, 4455–4460 (2007).
Sancho, R. et al. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J. 28, 1843–1854 (2009).
Acknowledgements
We are grateful to N. Ahn, M. Cobb and A. Hock for providing reagents. We thank B. Spencer-Dean, E. Nye and R. Mitter for technical support; the Molecular Biology Core Facility, Paterson Institute for Cancer Research, Manchester, UK for microarray analysis; and H. Walden for critical reading of the manuscript. The London Research Institute is funded by Cancer Research UK.
Author information
Authors and Affiliations
Contributions
C.D and A.C performed all experiments; C.D and A.B designed and analysed all experiments and wrote the manuscript; K.H and J.H generated R26RACO−1 transgenic mice; F.C generated RACO-1 antibodies.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2003 kb)
Rights and permissions
About this article
Cite this article
Davies, C., Chakraborty, A., Cipriani, F. et al. Identification of a co-activator that links growth factor signalling to c-Jun/AP-1 activation. Nat Cell Biol 12, 963–972 (2010). https://doi.org/10.1038/ncb2098
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2098
This article is cited by
-
Vγ1 and Vγ4 gamma-delta T cells play opposing roles in the immunopathology of traumatic brain injury in males
Nature Communications (2023)
-
Regulation of P53 signaling in breast cancer by the E3 ubiquitin ligase RNF187
Cell Death & Disease (2022)
-
Regulation of Hippo signaling and triple negative breast cancer progression by an ubiquitin ligase RNF187
Oncogenesis (2020)
-
An essential role of RNF187 in Notch1 mediated metastasis of hepatocellular carcinoma
Journal of Experimental & Clinical Cancer Research (2019)
-
RNF187 is Downregulated Following NF-κB Inhibition in Late Erythroblasts
Biochemical Genetics (2016)