Abstract
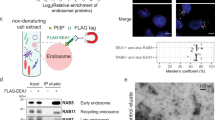
γ-Secretase, an aspartyl protease that belongs to the iCLiPs (intramembrane cleaving proteases) family, is a multiprotein complex that consists of presenilin (PS), nicastrin (NCT), Aph-1 and Pen-2 (ref. 1). It is responsible for generation of the β-amyloid peptide (Aβ), the primary component of senile plaques in the brains of patients with Alzheimer's disease. Although the four components are necessary and sufficient for γ-secretase activity2,3,4, additional proteins are possibly involved in its regulation. Consequently, we purified proteins associated with the active γ-secretase complex from reconstituted PS-deficient fibroblasts, using tandem affinity purification (TAP)5 and identified a series of proteins that transiently interact with the γ-secretase complex and are probably involved in complex maturation, membrane trafficking and, importantly, the tetraspanin web. Tetraspanins form detergent-resistant microdomains in the cell membrane and regulate cell adhesion, cell signalling and proteolysis6,7. Association of the γ-secretase complex with tetraspanin-enriched microdomains provides an explanation for the previously documented localization of γ-secretase to raft-like domains8. Thus, these studies suggest that maintenance of the integrity of tetraspanin microdomains contributes to the refinement of proteolytic activity of the γ-secretase complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
De Strooper, B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active γ-secretase complex. Neuron 38, 9–12 (2003).
Edbauer, D. et al. Reconstitution of γ-secretase activity. Nature Cell Biol. 5, 486–488 (2003).
Kimberly, W. T. et al. γ-Secretase is a membrane protein complex comprised of presenilin, nicastrin, aph-1, and pen-2. Proc. Natl Acad. Sci. USA 100, 6382–6387 (2003).
Takasugi, N. et al. The role of presenilin cofactors in the γ-secretase complex. Nature 422, 438–441 (2003).
Gavin, A. C. et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415, 141–147 (2002).
Hemler, M. E. Targeting of tetraspanin proteins—potential benefits and strategies. Nature Rev. Drug Discov. 7, 747–758 (2008).
Charrin, S. et al. Lateral organization of membrane proteins: tetraspanins spin their web. Biochem J. 420, 133–154 (2009).
Wahrle, S. et al. Cholesterol-dependent γ-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol. Dis. 9, 11–23 (2002).
Annaert, W. & De Strooper, B. A cell biological perspective on Alzheimer's Disease. Annu. Rev. Cell Dev. Biol. 18, 25–51 (2002).
De Strooper, B. et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391, 387–390 (1998).
Wolfe, M. S. et al. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature 398, 513–517 (1999).
Chavez-Gutierrez, L. et al. Glu(332) in the Nicastrin ectodomain is essential for γ-secretase complex maturation but not for its activity. J. Biol. Chem. 283, 20096–20105 (2008).
Chen, F. et al. TMP21 is a presenilin complex component that modulates γ-secretase but not epsilon-secretase activity. Nature 440, 1208–1212 (2006).
Zhou, S., Zhou, H., Walian, P. J. & Jap, B. K. CD147 is a regulatory subunit of the γ-secretase complex in Alzheimer's disease amyloid β-peptide production. Proc. Natl Acad. Sci. USA 102, 7499–7504 (2005).
Parks, A. L. & Curtis, D. Presenilin diversifies its portfolio. Trends Genet. 23, 140–150 (2007).
Fraering, P. C. et al. Purification and characterization of the human γ-secretase complex. Biochemistry 43, 9774–9789 (2004).
Winkler, E. et al. Purification, pharmacological modulation, and biochemical characterization of interactors of endogenous human γ-secretase. Biochemistry 48, 1183–1197 (2009).
Bouwmeester, T. et al. A physical and functional map of the human TNF-α/NF-kappa B signal transduction pathway. Nature Cell Biol. 6, 97–105 (2004).
Ponting, C. P. et al. Identification of a novel family of presenilin homologues. Hum. Mol. Genet. 11, 1037–1044 (2002).
Weihofen, A., Binns, K., Lemberg, M. K., Ashman, K. & Martoglio, B. Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science 296, 2215–2218 (2002).
Bentahir, M. et al. Presenilin clinical mutations can affect γ-secretase activity by different mechanisms. J. Neurochem. 96, 732–742 (2006).
Esler, W. P. et al. Activity-dependent isolation of the presenilin–γ-secretase complex reveals nicastrin and a γ substrate. Proc. Natl Acad. Sci. USA 99, 2720–2725 (2002).
Hansson, C. A. et al. Nicastrin, presenilin, APH-1, and PEN-2 form active γ-secretase complexes in mitochondria. J. Biol. Chem. 279, 51654–51660 (2004).
Boucheix, C. & Rubinstein, E. Tetraspanins. Cell. Mol. Life Sci. 58, 1189–1205 (2001).
Kolesnikova, T. V., Mannion, B. A., Berditchevski, F. & Hemler, M. E. β1 integrins show specific association with CD98 protein in low density membranes. BMC Biochem. 2, 10 (2001).
Claas, C., Stipp, C. S. & Hemler, M. E. Evaluation of prototype transmembrane 4 superfamily protein complexes and their relation to lipid rafts. J. Biol. Chem. 276, 7974–7984 (2001).
Ehehalt, R., Keller, P., Haass, C., Thiele, C. & Simons, K. Amyloidogenic processing of the Alzheimer β-amyloid precursor protein depends on lipid rafts. J. Cell Biol. 160, 113–123 (2003).
Cheng, H. et al. S-palmitoylation of γ-secretase subunits nicastrin and APH-1. J. Biol. Chem. 284, 1373–1384 (2009).
Hemler, M. E. Tetraspanin functions and associated microdomains. Nature Rev. Mol. Cell Biol. 6, 801–811 (2005).
Arduise, C. et al. Tetraspanins regulate ADAM10-mediated cleavage of TNF-α and epidermal growth factor. J. Immunol. 181, 7002–7013 (2008).
Silvie, O. et al. Cholesterol contributes to the organization of tetraspanin-enriched microdomains and to CD81-dependent infection by malaria sporozoites. J. Cell Sci. 119, 1992–2002 (2006).
Lavens, D. et al. A complex interaction pattern of CIS and SOCS2 with the leptin receptor. J. Cell Sci. 119, 2214–2224 (2006).
Acknowledgements
We thank T. E. Golde (Mayo Clinic, FL, US) for SPPL3 cDNA, S. Aerts (VIB), E. Karran and the bioinformatics team of Elli-Lilly for helpful discussion and A. Thathiah for critical reading of the manuscript. This work was supported by a Pioneer award from the Alzheimer's Association, the Fund for Scientific Research, Flanders; Katholieke Universiteit Leuven (GOA); Federal Office for Scientific Affairs (IUAP P6/58) and a Methusalem grant from the Flemisch government. The laboratory in Ghent is supported by research grants from the Fund for Scientific Research – Flanders (Belgium), the Concerted Research Actions (GOA) from the Ghent University, the Inter University Attraction Poles (IUAP06) and the European Union Interaction Proteome (6th Framework Program). L.B. is fellow of the FWO (Fonds voor Wetenschappelijk Onderzoek) and T.W. was fellow of the Canon Foundation in Europe and Marie Curie program of the EC.
Author information
Authors and Affiliations
Contributions
T.W. designed the concept and performed most of the experiments, interpreted the data and helped write the manuscript. K.C. and L.B. performed experiments, interpreted the data and helped write the manuscript. M.B. performed initial experiments, generated cell lines, performed initial purifications of TAP-tagged proteins and helped write the manuscript. F.B. and P.H. created the γ-secretase inhibitor column, participated in discussions and helped write the manuscript. A.S. and E.T. performed LC-MS/MS experiments and analysed the results. J.V. and K.G. performed LC-MS/MS experiments and interpreted these results, supervised this part of the research and helped write the manuscript. E.C. and C.B. participated in discussions, provided advice on tetraspanin experiments and tools, and helped write the manuscript. B.D.S. designed the concept and experiments, supervised the research, coordinated experiments, interpreted the results and helped write the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1596 kb)
Supplementary Information
Supplementary Table 1 (XLS 24 kb)
Supplementary Information
Supplementary Table 2 (XLS 41 kb)
Rights and permissions
About this article
Cite this article
Wakabayashi, T., Craessaerts, K., Bammens, L. et al. Analysis of the γ-secretase interactome and validation of its association with tetraspanin-enriched microdomains. Nat Cell Biol 11, 1340–1346 (2009). https://doi.org/10.1038/ncb1978
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1978
This article is cited by
-
Integrative analysis of cell adhesion molecules in glioblastoma identified prostaglandin F2 receptor inhibitor (PTGFRN) as an essential gene
BMC Cancer (2022)
-
γ-Secretase in Alzheimer’s disease
Experimental & Molecular Medicine (2022)
-
The innate immunity protein IFITM3 modulates γ-secretase in Alzheimer’s disease
Nature (2020)
-
The APMAP interactome reveals new modulators of APP processing and beta-amyloid production that are altered in Alzheimer’s disease
Acta Neuropathologica Communications (2019)
-
Gamma-secretase-dependent signaling of receptor tyrosine kinases
Oncogene (2019)