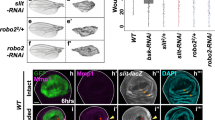
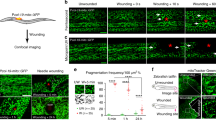
Abstract
Epidermal injury initiates a cascade of inflammation, epithelial remodelling and integument repair at wound sites. The regeneration of the extracellular barrier and damaged tissue repair rely on the precise orchestration of epithelial responses triggered by the injury1,2. Grainy head (Grh) transcription factors induce gene expression to crosslink the extracellular barrier in wounded flies and mice3,4. However, the activation mechanisms and functions of Grh factors in re-epithelialization remain unknown. Here we identify stitcher (stit), a new Grh target in Drosophila melanogaster. stit encodes a Ret-family receptor tyrosine kinase required for efficient epidermal wound healing. Live imaging analysis reveals that Stit promotes actin cable assembly during wound re-epithelialization. Stit activation also induces extracellular signal-regulated kinase (ERK) phosphorylation along with the Grh-dependent expression of stit and barrier repair genes at the wound sites. The transcriptional stimulation of stit on injury triggers a positive feedback loop increasing the magnitude of epithelial responses. Thus, Stit activation upon wounding coordinates cytoskeletal rearrangements and the level of Grh-mediated transcriptional wound responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schafer, M. & Werner, S. Transcriptional control of wound repair. Annu. Rev. Cell Dev. Biol. 23, 69–92 (2007).
Werner, S. & Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83, 835–870 (2003).
Mace, K. A., Pearson, J. C. & McGinnis, W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science 308, 381–385 (2005).
Ting, S. B. et al. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science 308, 411–413 (2005).
Harden, N. Cell biology. Of grainy heads and broken skins. Science 308, 364–365 (2005).
Stramer, B. & Martin, P. Cell biology: master regulators of sealing and healing. Curr. Biol. 15, R425–R427 (2005).
Uv, A. E., Harrison, E. J. & Bray, S. J. Tissue-specific splicing and functions of the Drosophila transcription factor Grainyhead. Mol. Cell. Biol. 17, 6727–6735 (1997).
Venkatesan, K., McManus, H. R., Mello, C. C., Smith, T. F. & Hansen, U. Functional conservation between members of an ancient duplicated transcription factor family, LSF/Grainyhead. Nucleic Acids Res. 31, 4304–4316 (2003).
Narasimha, M., Uv, A., Krejci, A., Brown, N. H. & Bray, S. J. Grainy head promotes expression of septate junction proteins and influences epithelial morphogenesis. J. Cell Sci. 121, 747–752 (2008).
Barolo, S., Carver, L. A. & Posakony, J. W. GFP and β-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques 29, 726, 728, 730, 732 (2000).
Bray, S. J. & Kafatos, F. C. Developmental function of Elf-1: an essential transcription factor during embryogenesis in Drosophila. Genes Dev. 5, 1672–1683 (1991).
Hemphala, J., Uv, A., Cantera, R., Bray, S. & Samakovlis, C. Grainy head controls apical membrane growth and tube elongation in response to Branchless/FGF signalling. Development 130, 249–258 (2003).
Edwards, K. A., Demsky, M., Montague, R. A., Weymouth, N. & Kiehart, D. P. GFP–moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev. Biol. 191, 103–117 (1997).
Chihara, T., Kato, K., Taniguchi, M., Ng, J. & Hayashi, S. Rac promotes epithelial cell rearrangement during tracheal tubulogenesis in Drosophila. Development 130, 1419–1428 (2003).
Morin, X., Daneman, R., Zavortink, M. & Chia, W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl Acad. Sci. USA 98, 15050–15055 (2001).
Wood, W. et al. Wound healing recapitulates morphogenesis in Drosophila embryos. Nature Cell Biol. 4, 907–912 (2002).
Runeberg-Roos, P. & Saarma, M. Neurotrophic factor receptor RET: structure, cell biology, and inherited diseases. Ann. Med. 39, 572–580 (2007).
Fantl, W. J., Johnson, D. E. & Williams, L. T. Signalling by receptor tyrosine kinases. Annu. Rev. Biochem. 62, 453–481 (1993).
Lee, T., Hacohen, N., Krasnow, M. & Montell, D. J. Regulated Breathless receptor tyrosine kinase activity required to pattern cell migration and branching in the Drosophila tracheal system. Genes Dev. 10, 2912–2921 (1996).
Duchek, P., Somogyi, K., Jekely, G., Beccari, S. & Rorth, P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107, 17–26 (2001).
Brunner, D. et al. A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell 76, 875–888 (1994).
Gabay, L., Seger, R. & Shilo, B. Z. MAP kinase in situ activation atlas during Drosophila embryogenesis. Development 124, 3535–3541 (1997).
McKay, M. M. & Morrison, D. K. Integrating signals from RTKs to ERK/MAPK. Oncogene 26, 3113–3121 (2007).
Martin, P. & Parkhurst, S. M. Parallels between tissue repair and embryo morphogenesis. Development 131, 3021–3034 (2004).
Zhao, M. et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature 442, 457–460 (2006).
Jacinto, A., Martinez-Arias, A. & Martin, P. Mechanisms of epithelial fusion and repair. Nature Cell Biol. 3, E117–E123 (2001).
Galko, M. J. & Krasnow, M. A. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2, E239 (2004).
Wenczak, B. A., Lynch, J. B. & Nanney, L. B. Epidermal growth factor receptor distribution in burn wounds. Implications for growth factor-mediated repair. J. Clin. Invest. 90, 2392–2401 (1992).
Chmielowiec, J. et al. c-Met is essential for wound healing in the skin. J. Cell Biol. 177, 151–162 (2007).
Gurtner, G. C., Werner, S., Barrandon, Y. & Longaker, M. T. Wound repair and regeneration. Nature 453, 314–321 (2008).
Tsarouhas, V. et al. Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev. Cell 13, 214–225 (2007).
Bement, W. M., Forscher, P. & Mooseker, M. S. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J. Cell Biol. 121, 565–578 (1993).
Acknowledgements
We thank W. McGinnis, S. Hayashi, S. Bray, P. Rørth, R. Hodgetts, the Bloomington, the Exelixis–Harvard Stock Centers, Developmental Studies Hybridoma Bank and Drosophila Genomics Resource Center for reagents; H. Jin, J. Hemphälä, M. Björk and I. Granel for technical assistance; and A. Uv, U. Theopold, R. Palmer, K.-A. Senti and colleagues at Wenner-Gren Institute for critical suggestions on the manuscript. Vetenskapsrådet, Cancerfonden and G. Gustafsson Stiftelsen provided financial support.
Author information
Authors and Affiliations
Contributions
M.G. and S.W. conducted the bioinformatics analysis. S.W. generated and analysed most strains, helped with live imaging and co-wrote the manuscript. V.T. performed and anlaysed the live imaging and wrote part of the manuscript. N.X. generated and analysed the kinase-dead stit strains and performed quantitative polymerase chain reaction and electrophoretic mobility-shift assays. N.S. conducted immunoprecipitation experiments. K.T. performed the electron microscopy analysis. N.N. generated the stitδ construct. C.S. designed the project, analysed results and co-wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information (PDF 1875 kb)
Supplementary Information
Supplementary Movie 1 (MOV 405 kb)
Supplementary Information
Supplementary Movie 2 (MOV 2507 kb)
Supplementary Information
Supplementary Movie 3 (MOV 404 kb)
Supplementary Information
Supplementary Movie 4 (MOV 958 kb)
Supplementary Information
Supplementary movie 5 (MOV 660 kb)
Rights and permissions
About this article
Cite this article
Wang, S., Tsarouhas, V., Xylourgidis, N. et al. The tyrosine kinase Stitcher activates Grainy head and epidermal wound healing in Drosophila. Nat Cell Biol 11, 890–895 (2009). https://doi.org/10.1038/ncb1898
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1898
This article is cited by
-
Sensing microbial infections in the Drosophila melanogaster genetic model organism
Immunogenetics (2022)
-
Hedgehog signaling regulates regenerative patterning and growth in Harmonia axyridis leg
Cellular and Molecular Life Sciences (2021)
-
Initiating a regenerative response; cellular and molecular features of wound healing in the cnidarian Nematostella vectensis
BMC Biology (2014)
-
The role of transcription-independent damage signals in the initiation of epithelial wound healing
Nature Reviews Molecular Cell Biology (2013)