Abstract
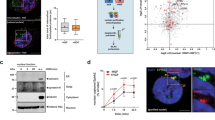
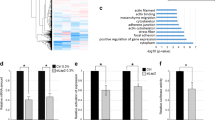
Rho GTPases control cytoskeletal dynamics through cytoplasmic effectors and regulate transcriptional activation through myocardin-related transcription factors (MRTFs), which are co-activators for serum response factor (SRF). We used RNA interference to investigate the contribution of the MRTF–SRF pathway to cytoskeletal dynamics in MDA-MB-231 breast carcinoma and B16F2 melanoma cells, in which basal MRTF–SRF activity is Rho-dependent. Depletion of MRTFs or SRF reduced cell adhesion, spreading, invasion and motility in culture, without affecting proliferation or inducing apoptosis. MRTF-depleted tumour cell xenografts showed reduced cell motility but proliferated normally. Tumour cells depleted of MRTF or SRF failed to colonize the lung from the bloodstream, being unable to persist after their arrival in the lung. Only a few genes show MRTF-dependent expression in both cell lines. Two of these, MYH9 (NMHCIIa) and MYL9 (MLC2), are also required for invasion and lung colonization. Conversely, expression of activated MAL/MRTF-A increases lung colonization by poorly metastatic B16F0 cells. Actin-based cell behaviour and experimental metastasis thus require Rho-dependent nuclear signalling through the MRTF–SRF network.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Geiger, B. & Bershadsky, A. Assembly and mechanosensory function of focal contacts. Curr. Opin. Cell Biol. 13, 584–592 (2001).
Sahai, E. & Marshall, C. J. RHO-GTPases and cancer. Nature Rev. Cancer 2, 133–142 (2002).
Yamaguchi, H. & Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta 1773, 642–652 (2007).
Clark, E. A., Golub, T. R., Lander, E. S. & Hynes, R. O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature 406, 532–535 (2000).
Croft, D. R. et al. Conditional ROCK activation in vivo induces tumor cell dissemination and angiogenesis. Cancer Res. 64, 8994–9001 (2004).
Hakem, A. et al. RhoC is dispensable for embryogenesis and tumor initiation but essential for metastasis. Genes Dev. 19, 1974–1979 (2005).
Suwa, H. et al. Overexpression of the rhoC gene correlates with progression of ductal adenocarcinoma of the pancreas. Br. J. Cancer 77, 147–152 (1998).
van Golen, K. L. et al. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin. Cancer Res. 5, 2511–2519 (1999).
Cen, B. et al. Megakaryoblastic leukemia 1, a potent transcriptional co-activator for serum response factor (SRF), is required for serum induction of SRF target genes. Mol. Cell Biol. 23, 6597–6608 (2003).
Miralles, F., Posern, G., Zaromytidou, A.-I. & Treisman, R. Actin dynamics control SRF activity by regulation of its co-activator MAL. Cell 113, 329–342 (2003).
Guettler, S., Vartiainen, M. K., Miralles, F., Larijani, B. & Treisman, R. RPEL motifs link the SRF cofactor MAL but not myocardin to Rho signalling via actin binding. Mol. Cell Biol. (2007).
Vartiainen, M. K., Guettler, S., Larijani, B. & Treisman, R. Nuclear actin regulates dynamic subcellular localization and activity of the SRF cofactor MAL. Science 316, 1749–1752 (2007).
Sun, Q. et al. Defining the mammalian CArGome. Genome Res. 16, 197–207 (2006).
Alberti, S. et al. Neuronal migration in the murine rostral migratory stream requires serum response factor. Proc. Natl Acad. Sci. USA 102, 6148–6153 (2005).
Schratt, G. et al. Serum response factor is crucial for actin cytoskeletal organization and focal adhesion assembly in embryonic stem cells. J. Cell Biol. 156, 737–750 (2002).
Somogyi, K. & Rorth, P. Evidence for tension-based regulation of Drosophila MAL and SRF during invasive cell migration. Dev. Cell 7, 85–93 (2004).
Knoll, B. et al. Serum response factor controls neuronal circuit assembly in the hippocampus. Nature Neurosci. 9, 195–204 (2006).
Kusama, T., Mukai, M., Tatsuta, M., Nakamura, H. & Inoue, M. Inhibition of transendothelial migration and invasion of human breast cancer cells by preventing geranylgeranylation of Rho. Int. J. Oncol. 29, 217–223 (2006).
Pille, J. Y. et al. Anti-RhoA and anti-RhoC siRNAs inhibit the proliferation and invasiveness of MDA-MB-231 breast cancer cells in vitro and in vivo. Mol. Ther. 11, 267–274 (2005).
Yoshioka, K., Foletta, V., Bernard, O. & Itoh, K. A role for LIM kinase in cancer invasion. Proc. Natl Acad. Sci. USA 100, 7247–7252 (2003).
Dua, P. & Gude, R. P. Pentoxifylline impedes migration in B16F10 melanoma by modulating Rho GTPase activity and actin organisation. Eur. J. Cancer 44, 1587–95 (2008).
Nakajima, M. et al. Effect of Wf-536, a novel ROCK inhibitor, against metastasis of B16 melanoma. Cancer Chemother. Pharmacol. 52, 319–324 (2003).
Caceres, M., Guerrero, J. & Martinez, J. Overexpression of RhoA–GTP induces activation of the Epidermal Growth Factor Receptor, dephosphorylation of focal adhesion kinase and increased motility in breast cancer cells. Exp. Cell Res. 309, 229–238 (2005).
Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001).
Tan, W., Martin, D. & Gutkind, J. S. The Gα13–Rho signaling axis is required for SDF-1-induced migration through CXCR4. J. Biol. Chem. 281, 39542–39549 (2006).
Gomes, E. R., Jani, S. & Gundersen, G. G. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell 121, 451–463 (2005).
Bhowmick, N. A., Neilson, E. G. & Moses, H. L. Stromal fibroblasts in cancer initiation and progression. Nature 432, 332–337 (2004).
Orimo, A. & Weinberg, R. A. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5, 1597–1601 (2006).
Gaggioli, C. et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nature Cell Biol. 9, 1392–1400 (2007).
Wang, W. et al. The activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumors. J. Cell Biol. 173, 395–404 (2006).
Xue, C. et al. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res. 66, 192–197 (2006).
Sahai, E. et al. Simultaneous imaging of GFP, CFP and collagen in tumors in vivo using multiphoton microscopy. BMC Biotechnol. 5, 14 (2005).
Bouzahzah, B. et al. Rho family GTPases regulate mammary epithelium cell growth and metastasis through distinguishable pathways. Mol. Med. 7, 816–830 (2001).
Morita, T., Mayanagi, T. & Sobue, K. Dual roles of myocardin-related transcription factors in epithelial mesenchymal transition via slug induction and actin remodeling. J. Cell Biol. 179, 1027–1042 (2007).
Clark, K., Langeslag, M., Figdor, C. G. & van Leeuwen, F. N. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol. 17, 178–186 (2007).
Somlyo, A. P. & Somlyo, A. V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G. proteins, kinases, and myosin phosphatase. Physiol. Rev. 83, 1325–1358 (2003).
Betapudi, V., Licate, L. S. & Egelhoff, T. T. Distinct roles of nonmuscle myosin II isoforms in the regulation of MDA-MB-231 breast cancer cell spreading and migration. Cancer Res. 66, 4725–4733 (2006).
Dulyaninova, N. G., House, R. P., Betapudi, V. & Bresnick, A. R. Myosin-IIA heavy-chain phosphorylation regulates the motility of MDA-MB-231 carcinoma cells. Mol. Biol. Cell 18, 3144–3155 (2007).
Wyckoff, J. B., Pinner, S. E., Gschmeissner, S., Condeelis, J. S. & Sahai, E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr. Biol. 16, 1515–1523 (2006).
Goulimari, P. et al. Galpha12/13 is essential for directed cell migration and localized Rho–Dia1 function. J. Biol. Chem. 280, 42242–42251 (2005).
Sotiropoulos, A., Gineitis, D., Copeland, J. & Treisman, R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell 98, 159–169 (1999).
Philippar, U. et al. The SRF target gene Fhl2 antagonizes RhoA/MAL-dependent activation of SRF. Mol. Cell 16, 867–880 (2004).
Selvaraj, A. & Prywes, R. Expression profiling of serum inducible genes identifies a subset of SRF target genes that are MKL dependent. BMC Mol. Biol. 5, 13 (2004).
Morita, T., Mayanagi, T. & Sobue, K. Reorganization of the actin cytoskeleton via transcriptional regulation of cytoskeletal/focal adhesion genes by myocardin-related transcription factors (MRTFs/MAL/MKLs). Exp. Cell Res. 313, 3432–3445 (2007).
Jiang, W. G. et al. Differential expression of the CCN family members Cyr61, CTGF and Nov in human breast cancer. Endocr. Relat. Cancer 11, 781–791 (2004).
Saez, E. et al. c-fos is required for malignant progression of skin tumors. Cell 82, 721–732 (1995).
Pinner, S. & Sahai, E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nature Cell Biol. 10, 127–137 (2008).
van de Wetering, M. et al. Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep. 4, 609–615 (2003).
Smyth, G. K. in Bioinformatics and Computational Biology Solutions using R. and Bioconductor (eds Gentleman, R., Carey, V., Dudoit, S., Irizarry, R. & Huber, W.) 397–420 (Springer, New York, 2005).
Li, S., Chang, S., Qi, X., Richardson, J. A. & Olson, E. N. Requirement of a myocardin-related transcription factor for development of mammary myoepithelial cells. Mol. Cell Biol. 26, 5797–5808 (2006).
Acknowledgements
We thank Hans Clevers for pTER, Richard Hynes for B16F2 and B16F0 cells. We thank Rob Nicolas for helpful discussions and continuous support, and other members of the Treisman and Sahai laboratories for technical help and discussions. We thank the Cancer Research UK Affymetrix Facility at the Paterson Institute for Cancer Research, in particular Yvonne Hey, and Gavin Kelly, Phill East, Richard Mitter at the CRUK Biostatistics Laboratory for the microarray processing and data analysis. We thank Clare Watkins, Emma Murray and Emma Nye (LRI Experimental Pathology laboratory), Daniel Zicha and Colin Gray (LRI light microscopy), and Ayad Eddaoudi and Derek Davies (LRI FACS laboratory) for expert and efficient technical support. S.M. was funded in part by a fellowship from the FRM (Fondation pour la Recherche Medicale). This work was funded by Cancer Research UK.
Author information
Authors and Affiliations
Contributions
S.M. designed the siRNA and ShRNAs and characterized the shRNA cell lines. S.M. designed, conducted and interpreted the wound-healing and adhesion experiments, reporter analyses, transcriptome analyses, the experimental metastasis experiments and the studies with activated MRTF-A; C.P-S. established the stable cell lines and conducted preliminary adhesion, motility and metastasis experiments; E.S. conducted the intravital microscopy, and advised on design of the experimental metastasis experiments; C.G. and S.M. conducted the organotypic invasion experiments. R.T. conceived the project, designed experiments and interpreted data. R.T. and S.M. wrote the manuscript, with additional input from E.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2514 kb)
Supplementary Information
Supplementary Table 1 (XLS 457 kb)
Supplementary Information
Supplementary Movie 1 (AVI 2568 kb)
Supplementary Information
Supplementary Movie 2 (AVI 2528 kb)
Supplementary Information
Supplementary Movie 3 (AVI 5806 kb)
Rights and permissions
About this article
Cite this article
Medjkane, S., Perez-Sanchez, C., Gaggioli, C. et al. Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nat Cell Biol 11, 257–268 (2009). https://doi.org/10.1038/ncb1833
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1833
This article is cited by
-
The benign nature and rare occurrence of cardiac myxoma as a possible consequence of the limited cardiac proliferative/ regenerative potential: a systematic review
BMC Cancer (2023)
-
PRPF19 facilitates colorectal cancer liver metastasis through activation of the Src-YAP1 pathway via K63-linked ubiquitination of MYL9
Cell Death & Disease (2023)
-
Interplay of the transcription factor MRTF-A and matrix stiffness controls mammary acinar structure and protrusion formation
Cell Communication and Signaling (2022)
-
RNA-binding proteins in ovarian cancer: a novel avenue of their roles in diagnosis and treatment
Journal of Translational Medicine (2022)
-
Lamina-associated polypeptide 2α is required for intranuclear MRTF-A activity
Scientific Reports (2022)