Abstract
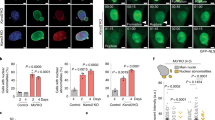
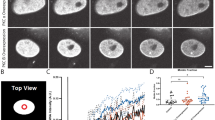
ING proteins interact with core histones through their plant homeodomains (PHDs)1,2,3,4 and with histone acetyltransferase (HAT) and histone deacetylase (HDAC) complexes to alter chromatin structure5,6,7. Here we identify a lamin interaction domain (LID) found only in ING proteins, through which they bind to and colocalize with lamin A. Lamin knockout (LMNA−/−) cells show reduced levels of ING1 that mislocalize. Ectopic lamin A expression increases ING1 levels and re-targets it to the nucleus to act as an epigenetic regulator6,8. ING1 lacking the LID does not interact with lamin A or affect apoptosis. In LMNA−/− cells, apoptosis is not affected by ING1. Mutation of lamin A results in several laminopathies, including Hutchinson-Gilford progeria syndrome (HGPS), a severe premature ageing disorder9. HGPS cells have reduced ING1 levels that mislocalize. Expression of LID peptides to block lamin A–ING1 interaction induces phenotypes reminiscent of laminopathies including HGPS9,10. These data show that targeting of ING1 to the nucleus by lamin A maintains ING1 levels and biological function. Known roles for ING proteins in regulating apoptosis and chromatin structure indicate that loss of lamin A–ING interaction may be an effector of lamin A loss, contributing to the HGPS phenotype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Shi, X. et al. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature 442, 96–99 (2006).
Pena, P. V. et al. Molecular mechanism of histone H3K4me3 recognition by plant homeodomain of ING2. Nature 442, 100–103 (2006).
Martin, D. G. et al. The Yng1p plant homeodomain finger is a methyl-histone binding module that recognizes lysine 4-methylated histone H3. Mol. Cell Biol. 26, 7871–7879. (2006).
Palacios, A. et al. Solution structure and NMR characterization of the binding to methylated histone tails of the plant homeodomain finger of the tumour suppressor ING4. FEBS Lett. 580, 6903–6908 (2006).
Young, D. Three yeast proteins related to the human candidate tumor suppressor p33ING1 are associated with histone acetyltransferase activities. Mol. Cell Biol. 20, 3807–3816 (2000).
Reinberg, D. Role of the Sin3-histone deacetylase complex in growth regulation by the candidate tumor suppressor p33 (ING1). Mol. Cell Biol. 22, 835–848 (2002).
Doyon, Y. C. et al. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol. Cell 21, 51–64 (2006).
Soliman, M. A. & Riabowol, K. After a decade of study-ING, a PHD for a versatile family of proteins. Trends Biochem Sci. 32, 509–519 (2007).
Eriksson, M. et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, 293–298 (2003).
Burke, B. & Stewart, CL. The laminopathies: the functional architecture of the nucleus and its contribution to disease. Ann. Rev Genomics Hum Genet. 7, 369–405 (2006).
He, G. H., Helbing, C. C., Wagner, M. J., Sensen, C. W. & Riabowol, K. Mol. Biol Evol. 22, 104–116 (2005).
Riabowol, K. Extension of the replicative life span of human diploid fibroblasts by inhibition of the p33ING1 candidate tumor suppressor. Mol. Cell Biol. 17, 2014–2019 (1997).
Pedeux, R. et al. ING2 regulates the onset of replicative senescence by induction of p300-dependent p53 acetylation. Mol. Cell Biol. 25, 6639–6648 (2005).
Scott, M. et al. UV-induced binding of ING1 to PCNA regulates the induction of apoptosis. J. Cell Sci. 114, 3455–3462 (2001).
Chin, M. Y, & Li, G. The novel tumor suppressor p33ING2 enhances nucleotide excision repair via inducement of histone H4 acetylation and chromatin relaxation. Cancer Res. 66, 1906–1911 (2006).
Zastrow, M. S., Vicek, S. & Wilson, K. L. Proteins that bind A-type lamins: integrating isolated clues. J. Cell Sci. 117, 979–987 (2004).
Taniura, H., Glass, C. & Gerace, L. A chromatin binding site in the tail domain of nuclear lamins that interacts with core histones. J. Cell Biol. 131, 33–44 (1995).
Ozaki, T. et al. Complex formation between lamin A and the retinoblastoma gene product: identification of the domain on lamin A required for its interaction. Oncogene 9, 2649–2653 (1994).
Johnson, B. R. et al. A-type lamins regulate retinoblastoma protein function by promoting subnuclear localization and preventing proteosomal degradation. Proc. Natl Acad Sci. USA 101, 9677–9682 (2004).
Helsens, K., Martens, L., Vandekerckhove, J. & Gevaert, K. MascotDatfile: an open-source library to fully parse and analyse MASCOT MS/MS search results. Proteomics. 7, 364–366 (2007).
Broers, J. L., Ramaekers, F. C., Bonne, G., Yaou, R. B. & Hutchison, C. J. Nuclear lamins: laminopathies and their role in premature ageing. Physiol Rev. 86, 967–1008 (2006).
Sullivan, T. et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 147, 913–920 (1999).
Goldman, R. D. et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. U. S.A 101, 8963–8968 (2004).
Coles, A. H. et al. Deletion of p37Ing1 in mice reveals a p53-independent role for Ing1 in the suppression of cell proliferation, apoptosis, and tumorigenesis. Cancer Res. 67, 2054–2061 (2007).
Vieyra, D. et al. Human ING1 Proteins Differentially Regulate Histone Acetylation. J. Biol Chem. 277, 29832–29839 (2002).
Taverna, S. D. et al. Yng1 PHD finger binding to H3 trimethylated at K4 promotes NuA3 HAT activity at K14 of H3 and transcription at a subset of targeted ORFs. Mol. Cell 24, 785–796 (2006).
Nouman, G. S. et al. Down regulation of nuclear expression of the p33 (ING1b) inhibitor of growth protein in invasive carcinoma of the breast. J. Clin Pathol. 56, 507–511 (2003).
Vieyra, D. et al. Ing1 isoforms differentially affect apoptosis in a cell age-dependent manner. Cancer Res. 62, 4445–4452 (2002).
Narita, M. et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003).
Shumaker, D. K. et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc. Natl. Acad. Sci. U. S. A 103, 8703–8708 (2006).
Broers, J. L. et al. Dynamics of the nuclear lamina as monitored by GFP-tagged A-type lamins. J. Cell Sci. 112, 3463–3475 (1999).
Moir, R. D, Yoon, M., Khoun, S. & Goldman, R. D. Nuclear Lamins A and B1: Different Pathways of Assembly during Nuclear Envelope Formation in Living Cells. J. Cell Biol. 151, 1155–1168 (2000).
Candelario, J., Sudhakar, S., Navarro, S., Reddy, S. & Comai, L. Perturbation of wild-type lamin A metabolism results in a progeroid pgenotype. Aging Cell 7, 355–367 (2008).
Lai, A. et al. RBP1 recruits the mSIN3-histone deacetylase complex to the pocket of retinoblastoma tumor suppressor family proteins found in limited discrete regions of the nucleus at growth arrest. Mol. Cell Biol. 21, 2918–32 (2001).
Walzak, A. A., Veldhoen, N, Feng, X, Riabowol, K. & Helbing, C. C. Expression profiles of mRNA transcript variants encoding the human inhibitor of growth tumor suppressor gene family in normal and neoplastic tissues. Exp. Cell Res. 314, 273–85 (2008).
Acknowledgements
We thank E. White for lamin constructs, J. Quarrie and D. Rancourt for ES cells, the SACRI Microscopy Facility for cell imaging, D. Boland and S. Law of the SAS for ING antibodies, D. Schreimer for LC/MS/MS advice and L. Robertson for help with flow cytometry. This study was supported by grants from the CIHR to K.R., from the NSERC to J.B.R. and to B.B. from the NIH. K.R. is a Scientist of the AHFMR, M.A.S. holds studentships from AHFMR and the Alberta Cancer Board (ACB) and H.S. and P.B. hold an ACB studentship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1374 kb)
Rights and permissions
About this article
Cite this article
Han, X., Feng, X., Rattner, J. et al. Tethering by lamin A stabilizes and targets the ING1 tumour suppressor. Nat Cell Biol 10, 1333–1340 (2008). https://doi.org/10.1038/ncb1792
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1792
This article is cited by
-
Nonlinear mechanics of lamin filaments and the meshwork topology build an emergent nuclear lamina
Nature Communications (2020)
-
INGs are potential drug targets for cancer
Journal of Cancer Research and Clinical Oncology (2017)
-
LincRNA-p21 acts as a mediator of ING1b-induced apoptosis
Cell Death & Disease (2015)
-
Defining the minimal peptide sequence of the ING1b tumour suppressor capable of efficiently inducing apoptosis
Cell Death Discovery (2015)
-
A minimal ING1b fragment that improves the efficacy of HDAC-based cancer cell killing
Cell Death & Disease (2015)