Abstract
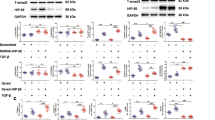
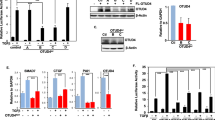
Transforming growth factor-β (TGF-β) is a multifunctional cytokine that regulates embryonic development and tissue homeostasis; however, aberrations of its activity occur in cancer1,2. TGF-β signals through its Type II and Type I receptors (TβRII and TβRI) causing phosphorylation of Smad proteins3,4. TGF-β-associated kinase 1 (TAK1), a member of the mitogen-activated protein kinase kinase kinase (MAPKKK) family, was originally identified as an effector of TGF-β-induced p38 activation5. However, the molecular mechanisms for its activation are unknown. Here we report that the ubiquitin ligase (E3) TRAF6 interacts with a consensus motif present in TβRI. The TβRI–TRAF6 interaction is required for TGF-β-induced autoubiquitylation of TRAF6 and subsequent activation of the TAK1–p38/JNK pathway, which leads to apoptosis. TβRI kinase activity is required for activation of the canonical Smad pathway, whereas E3 activity of TRAF6 regulates the activation of TAK1 in a receptor kinase-independent manner. Intriguingly, TGF-β-induced TRAF6-mediated Lys 63-linked polyubiquitylation of TAK1 Lys 34 correlates with TAK1 activation. Our data show that TGF-β specifically activates TAK1 through interaction of TβRI with TRAF6, whereas activation of Smad2 is not dependent on TRAF6.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Derynck R. & Zhang, Y. E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 425, 577–584 (2003).
Moustakas, A. & Heldin, C. H. Non-Smad TGF-β signals. J. Cell Sci. 118, 3573–3584 (2005).
Heldin, C. H., Miyazono, K. & ten Dijke, P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390, 465–471 (1997).
Shi, Y. & Massagué, J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113, 685–700 (2003).
Yamaguchi, K. et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-β signal transduction. Science 270, 2008–2011 (1995).
Groppe, J. et al. Cooperative assembly of TGF-β superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol. Cell 29, 157–168 2008.
Takatsu, Y. et al. TAK1 participates in c-Jun N-terminal kinase signaling during Drosophila development. Mol. Cell Biol. 9, 3015–3026 (2000).
Zhang, D. et al. TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nature Med. 5, 556–563 (2000).
Shibuya, H. et al. Role of TAK1 and TAB1 in BMP signalling in early Xenopus development EMBO J. 17, 1019–1028 (1998).
Chen, Z. J. Ubiquitin signalling in the NF-κB pathway. Nature Cell Biol. 8, 758–765 (2005).
Gao, M. & Karin, M. Regulating the regulators: control of protein ubiquitination and ubiquitin-like modifications by extracellular stimuli. Mol. Cell 19, 581–593 (2005).
Wu, H. & Arron, J. R. TRAF6, a molecular bridge spanning adaptive immunity, innate immunity and osteoimmunology. Bioessays 11, 1096–1105 (2003).
Deng, L. et al. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103, 351–361 (2000).
Lamothe, B. et al. Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 auto-ubiquitination is a critical determinant of IκB kinase activation. J. Biol. Chem. 282, 4102–4112 (2007).
Ninomiya-Tsuji, J. et al. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398, 252–256 (1999).
Kanayama, A. et al. TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol. Cell 15, 535–548 (2004).
Haglund, K., et al. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nature Cell Biol. 5, 461–546 (2003).
Watkins, S. J., Jonker, L. & Arthur, H. M. A direct interaction between TGFβ activated kinase 1 and the TGFβ type II receptor: implications for TGFβ signalling and cardiac hypertrophy. Cardiovasc. Res. 69, 432–439 (2006).
Weiss, A. & Schlessinger, J. Switching signals on or off by receptor dimerization. Cell 94, 277–280 (1998).
Darnay, B. G., Ni, J., Moore, P. A. & Aggarwal, B. B. Activation of NF-κB by RANK requires tumor necrosis factor receptor-associated factor (TRAF) 6 and NF-κB-inducing kinase. Identification of a novel TRAF6 interaction motif. J. Biol. Chem. 274, 7724–7731 (1999).
Pullen, S. S., Dang, T. T., Crute, J. J. & Kehry, M. R. CD40 signaling through tumor necrosis factor receptor-associated factors (TRAFs). Binding site specificity and activation of downstream pathways by distinct TRAFs. J. Biol. Chem. 274, 14246–14254 (1999).
Brown, K. et al. Structural basis for the interaction of TAK1 kinase with its activating protein TAB1. J. Mol. Biol. 354, 1013–1020 (2005).
Catic, A., Collins, C., Church, G. M. & Ploegh, H. L. Preferred in vivo ubiquitination sites. Bioinformatics 20, 3302–3307 (2004).
Wang, C et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature 412, 346–351 (2001).
Edlund, S. et al. TGF-β1-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TAK1 and MKK3. Mol. Biol. Cell 2, 529–544 (2003).
Zhang, S. et al. TGF-β1 induced activation of ATM and p53 mediates apoptosis in a Smad7-dependent manner. Cell Cycle 5, 2787–2795, (2006).
Franzén, P. et al. Cloning of a TGFβ type I receptor that forms a heteromeric complex with the TGFβ type II receptor. Cell 75, 681–692 (1993).
Acknowledgements
We are grateful to P. ten Dijke, K. Miyazono, I. Dikic, K. Matsumoto, J. Ninomiya-Tsuji, Z.J. Chen and V.M. Dixit for providing expression vectors and reagents, and J. Inoue for his gift of wild-type and TRAF6−/− MEFs. We are grateful to U. Engström who synthesized the p-TAK1 peptide used for generation of rabbit anti-serum. We thank J. Ericsson for his valuable advice on how to perform in vitro ubiquitylation assays, and A. Moustakas and A. Morén for their advice on how to perform in vivo ubiquitylation assays. This work was supported in part by grants from the Swedish Medical Research Council, the Swedish Cancer Society, Wenner-Grenska Society and the Torsten and Ragnar Söderbergs Foundation (M.L.).
Author information
Authors and Affiliations
Contributions
A.S., N.T., S.G., A.M., V.v.B., N.S., S.Z. and M.L. performed the experiments; C.-H.H. and M.L. planned the project; A.S., N.T., C.-H.H. and M.L. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4, S5 and S6 (PDF 904 kb)
Rights and permissions
About this article
Cite this article
Sorrentino, A., Thakur, N., Grimsby, S. et al. The type I TGF-β receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol 10, 1199–1207 (2008). https://doi.org/10.1038/ncb1780
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1780
This article is cited by
-
Cholesterol modulates type I/II TGF-β receptor complexes and alters the balance between Smad and Akt signaling in hepatocytes
Communications Biology (2024)
-
SMAD7 expression in CAR-T cells improves persistence and safety for solid tumors
Cellular & Molecular Immunology (2024)
-
Linking NLRP3 inflammasome and pulmonary fibrosis: mechanistic insights and promising therapeutic avenues
Inflammopharmacology (2023)
-
Importance of Fibrosis in the Pathogenesis of Uterine Leiomyoma and the Promising Anti-fibrotic Effects of Dipeptidyl Peptidase-4 and Fibroblast Activation Protein Inhibitors in the Treatment of Uterine Leiomyoma
Reproductive Sciences (2023)
-
TGF-beta signal transduction: biology, function and therapy for diseases
Molecular Biomedicine (2022)