Abstract
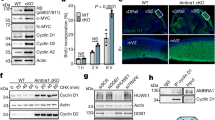
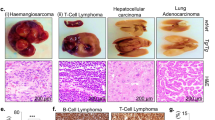
The anaphase promoting complex or cyclosome (APC/C) is a ubiquitin protein ligase that, together with Cdc20 or Cdh1, targets cell-cycle proteins for degradation. APC/C–Cdh1 specifically promotes protein degradation in late mitosis and G1. Mutant embryos lacking Cdh1 die at E9.5–E10.5 due to defects in the endoreduplication of trophoblast cells and placental malfunction. This lethality is prevented when Cdh1 is expressed in the placenta. Cdh1-deficient cells proliferate inefficiently and accumulate numeric and structural chromosomal aberrations, indicating that Cdh1 contributes to the maintenance of genomic stability. Cdh1 heterozygous animals show increased susceptibility to spontaneous tumours, suggesting that Cdh1 functions as a haploinsufficient tumour suppressor. These heterozygous mice also show several defects in behaviour associated with increased proliferation of stem cells in the nervous system. These results indicate that Cdh1 is required for preventing unscheduled proliferation of specific progenitor cells and protecting mammalian cells from genomic instability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Reed, S.I. Ratchets and clocks: the cell cycle, ubiquitylation and protein turnover. Nature Rev. Mol. Cell Biol. 4, 855–864 (2003).
Nakayama, K.I. & Nakayama, K. Ubiquitin ligases: cell-cycle control and cancer. Nature Rev. Cancer 6, 369–381 (2006).
Cardozo, T. & Pagano, M. The SCF ubiquitin ligase: insights into a molecular machine. Nature Rev. Mol. Cell Biol. 5, 739–751 (2004).
Harper, J.W., Burton, J.L. & Solomon, M.J. The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev. 16, 2179–2206 (2002).
Peters, J.M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nature Rev. Mol. Cell Biol. 7, 644–656 (2006).
Acquaviva, C. & Pines, J. The anaphase-promoting complex/cyclosome: APC/C. J. Cell Sci. 119, 2401–2404 (2006).
Pines, J. Mitosis: a matter of getting rid of the right protein at the right time. Trends Cell Biol. 16, 55–63 (2006).
Sullivan, M. & Morgan, D.O. Finishing mitosis, one step at a time. Nature Rev. Mol. Cell Biol. 8, 894–903 (2007).
Bashir, T., Dorrello, N.V., Amador, V., Guardavaccaro, D. & Pagano, M. Control of the SCF(Skp2–Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 428, 190–193 (2004).
Wei, W. et al. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature 428, 194–198 (2004).
Vodermaier, H.C. APC/C and SCF: controlling each other and the cell cycle. Curr. Biol. 14, R787–R796 (2004).
Ang, X.L. & Harper, J.W. Interwoven ubiquitination oscillators and control of cell cycle transitions. Sci. STKE 2004, pe31 (2004).
Carter, S.L., Eklund, A.C., Kohane, I.S., Harris, L.N. & Szallasi, Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nature Genet. 38, 1043–1048 (2006).
Cross, J.C. How to make a placenta: mechanisms of trophoblast cell differentiation in mice--a review. Placenta 26 (suppl. A), S3–S9 (2005).
Hayashi, S., Lewis, P., Pevny, L. & McMahon, A.P. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech. Dev. 119 (suppl 1), S97–S101 (2002).
Prinz, S., Hwang, E.S., Visintin, R. & Amon, A. The regulation of Cdc20 proteolysis reveals a role for APC components Cdc23 and Cdc27 during S phase and early mitosis. Curr. Biol. 8, 750–760 (1998).
Shirayama, M., Zachariae, W., Ciosk, R. & Nasmyth, K. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 17, 1336–1349 (1998).
Gieffers, C., Peters, B.H., Kramer, E.R., Dotti, C.G. & Peters, J.M. Expression of the CDH1-associated form of the anaphase-promoting complex in postmitotic neurons. Proc. Natl Acad. Sci. USA 96, 11317–11322 (1999).
Konishi, Y., Stegmuller, J., Matsuda, T., Bonni, S. & Bonni, A. Cdh1–APC controls axonal growth and patterning in the mammalian brain. Science 303, 1026–1030 (2004).
Alvarez-Buylla, A. & Garcia-Verdugo, J.M. Neurogenesis in adult subventricular zone. J. Neurosci. 22, 629–634 (2002).
Ferri, A.L. et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 131, 3805–3819 (2004).
Visintin, R., Prinz, S. & Amon, A. CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science 278, 460–463 (1997).
Schwab, M., Lutum, A.S. & Seufert, W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell 90, 683–693 (1997).
Yamaguchi, S., Murakami, H. & Okayama, H. A WD repeat protein controls the cell cycle and differentiation by negatively regulating Cdc2/B-type cyclin complexes. Mol. Biol. Cell 8, 2475–2486 (1997).
Kitamura, K., Maekawa, H. & Shimoda, C. Fission yeast Ste9, a homolog of Hct1/Cdh1 and Fizzy-related, is a novel negative regulator of cell cycle progression during G1-phase. Mol. Biol. Cell 9, 1065–1080 (1998).
Fay, D.S., Keenan, S. & Han, M. fzr-1 and lin-35/Rb function redundantly to control cell proliferation in C. elegans as revealed by a nonbiased synthetic screen. Genes Dev. 16, 503–517 (2002).
Sigrist, S.J. & Lehner, C.F. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell 90, 671–681 (1997).
Strausfeld, U.P. et al. Both cyclin A and cyclin E have S-phase promoting (SPF) activity in Xenopus egg extracts. J. Cell Sci. 109 (Pt 6), 1555–1563 (1996).
Moore, J.D., Kirk, J.A. & Hunt, T. Unmasking the S-phase-promoting potential of cyclin B1. Science 300, 987–990 (2003).
Engelbert, D., Schnerch, D., Baumgarten, A. & Wasch, R. The ubiquitin ligase APC(Cdh1) is required to maintain genome integrity in primary human cells. Oncogene 27, 907–917 (2008).
Sudo, T. et al. Activation of Cdh1-dependent APC is required for G1 cell cycle arrest and DNA damage-induced G2 checkpoint in vertebrate cells. EMBO J. 20, 6499–6508 (2001).
Perez de Castro, I., de Carcer, G. & Malumbres, M. A census of mitotic cancer genes: new insights into tumor cell biology and cancer therapy. Carcinogenesis 28, 899–912 (2007).
Karakaidos, P. et al. Overexpression of the replication licensing regulators hCdt1 and hCdc6 characterizes a subset of non-small-cell lung carcinomas: synergistic effect with mutant p53 on tumor growth and chromosomal instability-evidence of E2F-1 transcriptional control over hCdt1. Am. J. Pathol. 165, 1351–1365 (2004).
Pinyol, M. et al. Unbalanced expression of licensing DNA replication factors occurs in a subset of mantle cell lymphomas with genomic instability. Int. J. Cancer 119, 2768–2774 (2006).
DePinho, R.A. The age of cancer. Nature 408, 248–254 (2000).
Weaver, B.A., Silk, A.D., Montagna, C., Verdier-Pinard, P. & Cleveland, D.W. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell 11, 25–36 (2007).
Wang, C.X., Fisk, B.C., Wadehra, M., Su, H. & Braun, J. Overexpression of murine fizzy-related (fzr) increases natural killer cell-mediated cell death and suppresses tumor growth. Blood 96, 259–263 (2000).
Almeida, A., Bolanos, J.P. & Moreno, S. Cdh1/Hct1–APC is essential for the survival of postmitotic neurons. J. Neurosci. 25, 8115–8121 (2005).
Nait-Oumesmar, B. et al. Activation of the subventricular zone in multiple sclerosis: evidence for early glial progenitors. Proc. Natl Acad. Sci. USA 104, 4694–4699 (2007).
Rodriguez, C.I. et al. High-efficiency deleter mice show that FLPe is an alternative to Cre–loxP. Nature Genet. 25, 139–140 (2000).
Schwenk, F., Baron, U. & Rajewsky, K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 23, 5080–5081 (1995).
Malumbres, M. et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 118, 493–504 (2004).
Mendez, J. & Stillman, B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol. Cell Biol. 20, 8602–8612 (2000).
Quereda, V., Martinalbo, J., Dubus, P., Carnero, A. & Malumbres, M. Genetic cooperation between p21Cip1 and INK4 inhibitors in cellular senescence and tumor suppression. Oncogene 26, 7665–7674 (2007).
Miquel, J. & Blasco, M. A simple technique for evaluation of vitality loss in aging mice, by testing their muscular coordination and vigor. Exp. Gerontol. 13, 389–396 (1978).
Bevins, R.A. & Besheer, J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nature Protoc. 1, 1306–1311 (2006).
Acknowledgements
We thank Gardenia Fresneda and Manuel Eguren for help with carcinogenic assays and molecular analysis; and Sheila Rueda and Blanca Velasco for their valuable help in the management of the mouse colony. We also thank Sagrario Ortega and the CNIO Transgenic Unit for their expertise in ES cell manipulations, members of the CNIO Comparative Pathology Unit for histological and pathological processing and the CNIO Cytogenetics Unit for their help with karyotype analysis. I.G.H. is supported by a Ramón y Cajal contract (Ministerio de Educación y Ciencia). E.M. is supported by an FIS fellowship (Ministerio de Sanidad). This work was supported by grants from the Association pour la Recherche contre le Cancer and the Région Aquitaine (to P.D.), Ministerio de Educación y Ciencia (SAF2004-05611 to I.G.H.; BFU2004-04886 to J.M.; BFU2005-03195 and GEN2003-20243-C08-05 to S.M.; and SAF2006-05186 to M.M.), the Consolider-Ingenio 2010 Programme (CSD2007-00015 to J.M. and S.M.; and CSD2007-00017 to M.M.), Comunidad de Madrid (OncoCycle Programme; S-BIO-0283-2006), Fundación Ramón Areces, and Fundación Médica Mutua Madrileña Automovilística (to M.M.); and Fundación Científica de la Asociación Española contra el Cáncer (to S.M. and M.M.).
Author information
Authors and Affiliations
Contributions
I.G.H and E.M. performed most of experiments and J.M. carried out the analysis of chromatin-bound proteins. P.D. and M.C. performed the histological and pathological analysis of the samples. M.M. and S.M. designed and supervised the study and M.M. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4, S5, Supplementary Tables S1, S2 and Supplementary Methods (PDF 4416 kb)
Rights and permissions
About this article
Cite this article
García-Higuera, I., Manchado, E., Dubus, P. et al. Genomic stability and tumour suppression by the APC/C cofactor Cdh1. Nat Cell Biol 10, 802–811 (2008). https://doi.org/10.1038/ncb1742
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1742
This article is cited by
-
SIRT1 ubiquitination is regulated by opposing activities of APC/C-Cdh1 and AROS during stress-induced premature senescence
Experimental & Molecular Medicine (2023)
-
SRC kinase-mediated signaling pathways and targeted therapies in breast cancer
Breast Cancer Research (2022)
-
Efficient terminal erythroid differentiation requires the APC/C cofactor Cdh1 to limit replicative stress in erythroblasts
Scientific Reports (2022)
-
Insufficiency of FZR1 disturbs HSC quiescence by inhibiting ubiquitin-dependent degradation of RUNX1 in aplastic anemia
Leukemia (2022)
-
Ubiquitin signaling in cell cycle control and tumorigenesis
Cell Death & Differentiation (2021)