Abstract
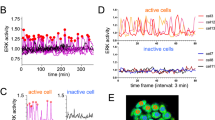
The restriction point (R-point) marks the critical event when a mammalian cell commits to proliferation and becomes independent of growth stimulation. It is fundamental for normal differentiation and tissue homeostasis, and seems to be dysregulated in virtually all cancers1,2. Although the R-point has been linked to various activities involved in the regulation of G1–S transition of the mammalian cell cycle2,3,4,5,6, the underlying mechanism remains unclear1,7. Using single-cell measurements, we show here that the Rb–E2F pathway functions as a bistable switch to convert graded serum inputs into all-or-none E2F responses. Once turned ON by sufficient serum stimulation, E2F can memorize and maintain this ON state independently of continuous serum stimulation. We further show that, at critical concentrations and duration of serum stimulation, bistable E2F activation correlates directly with the ability of a cell to traverse the R-point.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Malumbres, M. & Barbacid, M. To cycle or not to cycle: a critical decision in cancer. Nature Rev. Cancer 1, 222–231 (2001).
Weinberg, R. A. The biology of cancer. (Garland Science, New York, 2007).
Sears, R. C. & Nevins, J. R. Signaling networks that link cell proliferation and cell fate. J. Biol. Chem. 277, 11617–11620 (2002).
Frolov, M. V. & Dyson, N. J. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J. Cell Sci. 117, 2173–2181 (2004).
Attwooll, C., Lazzerini Denchi, E. & Helin, K. The E2F family: specific functions and overlapping interests. EMBO J. 23, 4709–4716 (2004).
Sherr, C. J. & Roberts, J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (1999).
Zetterberg, A., Larsson, O. & Wiman, K. G. What is the restriction point? Curr. Opin. Cell Biol. 7, 835–842 (1995).
Pardee, A. B. A restriction point for control of normal animal cell proliferation. Proc. Natl Acad. Sci. USA 71, 1286–1290 (1974).
Zetterberg, A. & Larsson, O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc. Natl Acad. Sci. USA 82, 5365–5369 (1985).
Xiong, W. & Ferrell, J. E. Jr., A positive-feedback-based bistable 'memory module' that governs a cell fate decision. Nature 426, 460–465 (2003).
Sha, W. et al. Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts. Proc. Natl Acad. Sci. USA 100, 975–980 (2003).
Ozbudak, E. M., Thattai, M., Lim, H. N., Shraiman, B. I. & Van Oudenaarden, A. Multistability in the lactose utilization network of Escherichia coli. Nature 427, 737–740 (2004).
Paliwal, S. et al. MAPK-mediated bimodal gene expression and adaptive gradient sensing in yeast. Nature 446, 46–51 (2007).
Aguda, B. D. & Tang, Y. The kinetic origins of the restriction point in the mammalian cell cycle. Cell Prolif. 32, 321–335 (1999).
Novak, B. & Tyson, J. J. A model for restriction point control of the mammalian cell cycle. J. Theoret. Biol. 230, 563–579 (2004).
Hatzimanikatis, V., Lee, K. H. & Bailey, J. E. A mathematical description of regulation of the G1–S transition of the mammalian cell cycle. Biotech. Bioeng. 65, 631–637 (1999).
Qu, Z., MacLellan, W. R. & Weiss, J. N. Dynamics of the cell cycle: checkpoints, sizers, and timers. Biophys. J. 85, 3600–3611 (2003).
Thron, C. D. Bistable biochemical switching and the control of the events of the cell cycle. Oncogene 15, 317–325 (1997).
Johnson, D. G., Schwarz, J. K., Cress, W. D. & Nevins, J. R. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature 365, 349–352 (1993).
Wu, L. et al. The E2F1-3 transcription factors are essential for cellular proliferation. Nature 414, 457–462 (2001).
Leung, J. Y., Ehmann, G. L., Giangrande, P. H. & Nevis, J. R. A role for Myc in facilitating transcription activation by E2F. Oncogene, advance online publication, doi:10.1038/onc.2008.55 (2008).
Tyson, J. J., Chen, K. C. & Novak, B. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr. Opin. Cell Biol. 15, 221–231 (2003).
Ferrell, Jr, J. E. . Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14, 140–148 (2002).
Gardner, T. S., Cantor, C. R. & Collins, J. J. Construction of a genetic toggle switch in Escherichia coli. Nature 403, 339–342 (2000).
Isaacs, F. J., Hasty, J., Cantor, C. R. & Collins, J. J. Prediction and measurement of an autoregulatory genetic module. Proc. Natl Acad. Sci. USA 100, 7714–7719 (2003).
Rao, C. V., Wolf, D. M. & Arkin, A. P. Control, exploitation and tolerance of intracellular noise. Nature 420, 231–237 (2002).
Longo, D. & Hasty, J. Dynamics of single-cell gene expression. Mol. Syst. Biol. 2, 64 (2006).
Bean, J. M., Siggia, E. D. & Cross, F. R. Coherence and timing of cell cycle start examined at single-cell resolution. Mol. Cell 21, 3–14 (2006).
Matsushime, H., Roussel, M. F., Ashmun, R. A. & Sherr, C. J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell 65, 701–713 (1991).
Dannenberg, J. H., van Rossum, A., Schuijff, L. & te Riele, H. Ablation of the retinoblastoma gene family deregulates G(1) control causing immortalization and increased cell turnover under growth-restricting conditions. Genes Dev. 14, 3051–3064 (2000).
Sage, J. et al. Targeted disruption of the three Rb-related genes leads to loss of G(1) control and immortalization. Genes Dev. 14, 3037–3050 (2000).
Acknowledgements
We thank W. Zhu, L. Kong, R. Rempel, T. Hallstrom, and C. Tan for comments on the manuscript. We also thank Y. Leung, S. Angus, E. Andrechek, Q. Wang, L. Jakoi, K. Culler and H. Zhang for their help. This project was supported by grants from the NIH 5-U24-CA112952-03 (to J.R.N.), NSF BES-0625213 (to L.Y.) and a David and Lucile Packard Fellowship (to L.Y.).
Author information
Authors and Affiliations
Contributions
G. Y., L. Y. and J. R. N. conceived the project; T. L., L. Y. and G. Y. performed the mathematical modelling; G. Y. performed the experiments. G. Y., L. Y. and J. R. N. analysed the data. S. M. contributed materials and reagents. G. Y., T. L., L. Y. and J. R. N wrote the paper.
Corresponding authors
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4, S5, S6, Supplementary Tables S1, S2, S3 and Supplementary Materials and Methods (PDF 2273 kb)
Rights and permissions
About this article
Cite this article
Yao, G., Lee, T., Mori, S. et al. A bistable Rb–E2F switch underlies the restriction point. Nat Cell Biol 10, 476–482 (2008). https://doi.org/10.1038/ncb1711
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1711
This article is cited by
-
SHR and SCR coordinate root patterning and growth early in the cell cycle
Nature (2024)
-
Unravelling how plant cells divide and differ
Nature (2024)
-
Commentary: locating the restriction point
Cell Division (2023)
-
Loss of CDK4/6 activity in S/G2 phase leads to cell cycle reversal
Nature (2023)
-
CDK4/6 initiates Rb inactivation and CDK2 activity coordinates cell-cycle commitment and G1/S transition
Scientific Reports (2022)