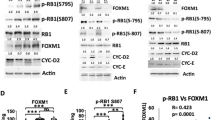
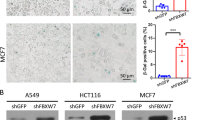
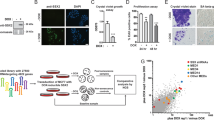
Abstract
Germline von Hippel–Lindau tumour suppressor gene (VHL) mutations cause renal cell carcinomas, haemangioblastomas and phaeochromocytomas in humans1. Mutations in VHL also occur in sporadic renal cell carcinomas. The protein encoded by VHL, VHL, is part of the ubiquitin ligase that downregulates the heterodimeric transcription factor Hif under well-oxygenated conditions1. Here we show that acute VHL inactivation causes a senescent-like phenotype in vitro and in vivo. This phenotype was independent of p53 and Hif but dependent on the retinoblastoma protein (Rb) and the SWI2/SNF2 chromatin remodeller p400. Rb activation occurred through a decrease in Skp2 messenger RNA, which resulted in the upregulation of p27 in a Hif-independent fashion. Our results suggest that senescence induced by VHL inactivation is a tumour-suppressive mechanism that must be overcome to develop VHL-associated neoplasias.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kaelin, W. G. Jr. Von Hippel–Lindau disease. Annu. Rev. Pathol. 2, 145–173.
Patil, C. K., Mian, I. S. & Campisi, J. The thorny path linking cellular senescence to organismal aging. Mech. Ageing Dev. 126, 1040–1045 (2005).
Lowe, S. W., Cepero, E. & Evan, G. Intrinsic tumour suppression. Nature 432, 307–315 (2004).
Di Leonardo, A., Linke, S. P., Clarkin, K. & Wahl, G. M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 8, 2540–2551 (1994).
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Collado, M. et al. Tumour biology: senescence in premalignant tumours. Nature 436, 642 (2005).
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005).
Haase, V. H., Glickman, J. N., Socolovsky, M. & Jaenisch, R. Vascular tumors in livers with targeted inactivation of the von Hippel–Lindau tumor suppressor. Proc. Natl Acad. Sci. USA 98, 1583–1588 (2001).
DeCaprio, J. A. et al. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell 54, 275–283 (1988).
Hayashi, S. & McMahon, A. P. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244, 305–318 (2002).
Li, L., et al. Hypoxia-inducible factor linked to differential kidney cancer risk seen with type 2A and type 2B VHL mutations. Mol. Cell. Biol. 27, 5381–5392.
Kondo, K., Kim, W. Y., Lechpammer, M. & Kaelin, W. G. Jr. Inhibition of HIF-2α is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 1, E83 (2003).
Kim, W. Y. et al. Failure to prolyl hydroxylate hypoxia-inducible factor α phenocopies VHL inactivation in vivo. EMBO J. 25, 4650–4662 (2006).
Sherr, C. J. & DePinho, R. A. Cellular senescence: mitotic clock or culture shock? Cell 102, 407–410.
Bowman, T. et al. Tissue-specific inactivation of p53 tumor suppression in the mouse. Genes Dev. 10, 826–835 (1996).
King, F. W., Wawrzynow, A., Hohfeld, J. & Zylicz, M. Co-chaperones Bag-1, Hop and Hsp40 regulate Hsc70 and Hsp90 interactions with wild-type or mutant p53. EMBO J. 20, 6297–6305 (2001).
Mack, F. A., Patel, J. H., Biju, M. P., Haase, V. H. & Simon, M. C. Decreased growth of Vhl−/− fibrosarcomas is associated with elevated levels of cyclin kinase inhibitors p21 and p27. Mol. Cell. Biol. 25, 4565–4578 (2005).
Gardner, L. B. et al. Hypoxia inhibits G1/S transition through regulation of p27 expression. J. Biol.Chem. 276, 7919–7926 (2001).
Goda, N. et al. Hypoxia-inducible factor 1α is essential for cell cycle arrest during hypoxia. Mol. Cell.Biol. 23, 259–269 (2003).
Carmeliet, P. et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 394, 485–490 (1998).
Amati, B. & Vlach, J. Kip1 meets SKP2: new links in cell-cycle control. Nature Cell Biol. 1, E91–E93 (1999).
Whyte, P. et al. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature 334, 124–129 (1988).
Samuelson, A. V. & Lowe, S. W. Selective induction of p53 and chemosensitivity in RB-deficient cells by E1A mutants unable to bind the RB-related proteins. Proc. Natl Acad. Sci. USA 94, 12094–12099 (1997).
Wang, H. G. et al. Identification of specific adenovirus E1A N-terminal residues critical to the binding of cellular proteins and to the control of cell growth. J. Virol. 67, 476–488 (1993).
Fuchs, M. et al. The p400 complex is an essential E1A transformation target. Cell 106, 297–307 (2001).
Samuelson, A. V. et al. p400 is required for E1A to promote apoptosis. J. Biol. Chem. 280, 21915–21923 (2005).
Chan, H. M., Narita, M., Lowe, S. W. & Livingston, D. M. The p400 E1A-associated protein is a novel component of the p53 → p21 senescence pathway. Genes Dev. 19, 196–201 (2005).
Welford, S. M. et al. HIF-1α delays premature senescence through the activation of MIF. Genes Dev. 20, 3366–3371 (2006).
Mandriota, S. J. et al. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell 1, 459–468 (2002).
Walther, M. M., Lubensky, I. A., Venzon, D., Zbar, B. & Linehan, W. M. Prevalence of microscopic lesions in grossly normal renal parenchyma from patients with von Hippel–Lindau disease, sporadic renal cell carcinoma and no renal disease: clinical implications. J. Urol. 154, 2010–2014 (1995).
Hayashi, S. & McMahon, A. P. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244, 305–318 (2002).
Masutomi, K. et al. Telomerase maintains telomere structure in normal human cells. Cell 114, 241–253 (2003).
Acknowledgements
This work was supported by the National Institutes of Health, HHMI, Doris Duke Foundation and the Murray Foundation.
Author information
Authors and Affiliations
Contributions
A. P. Y. and S. Schlisio designed the experiments; A. P. Y., S. Schlisio, Y. A. M., Q. Z., L. L. and C. G. performed the experiments; S. Signoretti analysed the data; A. P. Y. and W. G. K. analysed the data and wrote the paper.
Corresponding author
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4, S5 and S6 (PDF 2345 kb)
Rights and permissions
About this article
Cite this article
Young, A., Schlisio, S., Minamishima, Y. et al. VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat Cell Biol 10, 361–369 (2008). https://doi.org/10.1038/ncb1699
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1699
This article is cited by
-
Impaired oxygen-sensitive regulation of mitochondrial biogenesis within the von Hippel–Lindau syndrome
Nature Metabolism (2022)
-
Loss of the tumor suppressor BTG3 drives a pro-angiogenic tumor microenvironment through HIF-1 activation
Cell Death & Disease (2020)
-
S-Phase Kinase-associated Protein-2 Rejuvenates Senescent Endothelial Progenitor Cells and Induces Angiogenesis in Vivo
Scientific Reports (2020)
-
HIF-1α and HIF-2α differently regulate tumour development and inflammation of clear cell renal cell carcinoma in mice
Nature Communications (2020)
-
Cellular senescence in renal ageing and disease
Nature Reviews Nephrology (2017)