Abstract
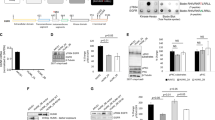
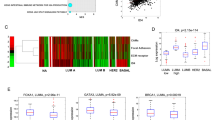
Epidermal growth factor (EGF) receptor (EGFR) signalling is implicated in tumour invasion and metastasis1,2. However, whether there are EGFR signalling pathways specifically used for tumour invasion still remains elusive. Overexpression of Arf6 and its effector, AMAP1, correlates with and is crucial for the invasive phenotypes of different breast cancer cells3,4,5,6. Here we identify the mechanism by which Arf6 is activated to induce tumour invasion. We found that GEP100/BRAG2, a guanine nucleotide exchanging factor (GEF) for Arf6, is responsible for the invasive activity of MDA-MB-231 breast cancer cells, whereas the other ArfGEFs are not. GEP100, through its pleckstrin homology domain, bound directly to Tyr1068/1086-phosphorylated EGFR to activate Arf6. Overexpression of GEP100, together with Arf6, caused non-invasive MCF7 cells7 to become invasive, which was dependent on EGF stimulation. Moreover, GEP100 knockdown blocked tumour metastasis. GEP100 was expressed in 70% of primary breast ductal carcinomas, and was preferentially co-expressed with EGFR in the malignant cases. Our results indicate that GEP100 links EGFR signalling to Arf6 activation to induce invasive activities of some breast cancer cells, and hence may contribute to their metastasis and malignancy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Blume-Jensen, P. & Hunter, T. Oncogenic kinase signalling. Nature 411, 355–365 (2001).
Hynes, N. & Lane, H. ERBB receptors and cancer: the complexity of targeted inhibitors. Nature Rev. Cancer 5, 341–354 (2005).
Hashimoto, S. et al. Requirement for Arf6 in breast cancer invasive activities. Proc. Natl Acad. Sci. USA 101, 6647–6652 (2004).
Onodera, Y. et al. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 24, 963–973 (2005).
Sabe, H. Requirement for Arf6 in cell adhesion, migration, and cancer cell invasion. J. Biochem. 134, 485–489 (2003).
Sabe, H., Onodera, Y., Mazaki, Y. & Hashimoto, S. ArfGAP family proteins in cell adhesion, migration and tumor invasion. Curr. Opin. Cell Biol. 18, 558–564 (2006).
Thompson, E. W. et al. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J. Cell Physiol. 150, 534–544 (1992).
Birchmeier, C., Birchmeier, W., Gherardi, E. & Vande Woude, G. F. Met, metastasis, motility and more. Nature Rev. Mol. Cell Biol. 4, 915–925 (2003).
Hashimoto, S. et al. Targeting AMAP1 and cortactin binding bearing an atypical src homology 3/proline interface for prevention of breast cancer invasion and metastasis. Proc. Natl Acad. Sci. USA 103, 7036–7041 (2006).
Cox, R., Mason-Gamer, R. J., Jackson, C. L. & Segev, N. Phylogenetic analysis of Sec7-domain-containing Arf nucleotide exchangers. Mol. Biol. Cell 15, 1487–1505 (2004).
D'Souza-Schorey, C. & Chavrier, P. ARF proteins: roles in membrane traffic and beyond. Nature Rev. Mol. Cell. Biol. 7, 347–358 (2006).
Someya, A. et al. ARF-GEP(100), a guanine nucleotide-exchange protein for ADP-ribosylation factor 6. Proc. Natl Acad. Sci. USA 98, 2413–2418 (2001).
Santy, L. C. & Casanova, J. E. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J. Cell Biol. 154, 599–610 (2001).
Derrien, V. et al. A conserved C-terminal domain of EFA6-family ARF6-guanine nucleotide exchange factors induces lengthening of microvilli-like membrane protrusions. J. Cell Sci. 115, 2867–2879 (2002).
Price, J. T., Tiganis, T., Agarwal, A., Djakiew, D. & Thompson, E. W. Epidermal growth factor promotes MDA-MB-231 breast cancer cell migration through a phosphatidylinositol 3′-kinase and phospholipase C-dependent mechanism. Cancer Res. 59, 5475–5478 (1999).
Honda, A. et al. Phosphatidylinositol 4-phosphate 5-kinase α is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 99, 521–532 (1999).
Zajchowski, D. et al. Expression of growth factors and oncogenes in normal and tumor-derived human mammary epithelial cells. Cancer Res. 48, 7041–7047 (1988).
Yamaji, R. et al. Identification and localization of two brefeldin A-inhibited guanine nucleotide-exchange proteins for ADP-ribosylation factors in a macromolecular complex. Proc. Natl Acad. Sci. USA 97, 2567–2572 (2000).
Di Paolo, G. & De Camilli, P. Phosphoinositides in cell regulation and membrane dynamics. Nature 443, 651–657 (2006).
Lemmon, M. A. & Ferguson, K. M. Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem. J. 350, 1–18 (2000).
Bowden, E. T., Barth, M., Thomas, D., Glazer, R. I. & Mueller, S. C. An invasion-related complex of cortactin, paxillin and PKCμ associates with invadopodia at sites of extracellular matrix degradation. Oncogene 18, 4440–4449 (1999).
Zajchowski, D. A. et al. Identification of gene expression profiles that predict the aggressive behavior of breast cancer cells. Cancer Res. 61, 5168–5178 (2001).
Fitzpatrick, S. L., LaChance, M. P. & Schultz, G. S. Characterization of epidermal growth factor receptor and action on human breast cancer cells in culture. Cancer Res. 44, 3442–3447 (1984).
Takeichi, M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251, 1451–1455 (1991).
Frixen, U. H. et al. E-cadherin-mediated cell–cell adhesion prevents invasiveness of human carcinoma cells. J. Cell Biol. 113, 173–185 (1991).
Condeelis, J. & Pollard, J. W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124, 263–266 (2006).
Rong, S. et al. Tumorigenicity of the met proto-oncogene and the gene for hepatocyte growth factor. Mol. Cell. Biol. 12, 5152–5158 (1992).
Silverstein, M. J. et al. Prognostic classification of breast ductal carcinoma-in-situ. Lancet 345, 1154–1157 (1995).
Toda, Y. et al. Application of tyramide signal amplification system to immunohistochemistry: a potent method to localize antigens that are not detectable by ordinary method. Pathol. Int. 49, 479–483 (1999).
Acknowledgements
We thank M. Hiraishi, M. Iwahara and M. Miyoshi for their help, T. Yoneda for 4T1/luc cells, RIKEN BioResource Center, M. Hirata and P. Randazzo for cDNAs, and H. A. Popiel for reading the manuscript. This work was supported in part by grants-in-aid from the Ministry of Education, Science, Sports and Culture of Japan, and by the Mochida Memorial Foundation and the Naito Foundation.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
Supplementry figures S1, S2, S3, S4, S5, S6 and Methods (PDF 618 kb)
Rights and permissions
About this article
Cite this article
Morishige, M., Hashimoto, S., Ogawa, E. et al. GEP100 links epidermal growth factor receptor signalling to Arf6 activation to induce breast cancer invasion. Nat Cell Biol 10, 85–92 (2008). https://doi.org/10.1038/ncb1672
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1672
This article is cited by
-
KRAS, MYC, and ARF6: inseparable relationships cooperatively promote cancer malignancy and immune evasion
Cell Communication and Signaling (2023)
-
ARF6 promotes hepatocellular carcinoma proliferation through activating STAT3 signaling
Cancer Cell International (2023)
-
Endocytosis in cancer and cancer therapy
Nature Reviews Cancer (2023)
-
DDR1 promotes hepatocellular carcinoma metastasis through recruiting PSD4 to ARF6
Oncogene (2022)
-
Targeting EGFR-dependent tumors by disrupting an ARF6-mediated sorting system
Nature Communications (2022)