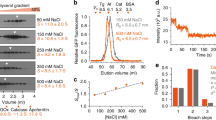
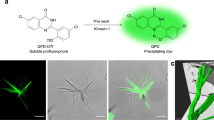
Abstract
Establishment and maintenance of cell structures and functions are highly dependent on the efficient regulation of intracellular transport in which proteins of the kinesin superfamily (KIFs) are very important. In this regard, how KIFs regulate the release of their cargo is a critical process that remains to be elucidated. To address this specific question, we have investigated the mechanism behind the regulation of the KIF17–Mint1 interaction. Here we report that the tail region of the molecular motor KIF17 is regulated by phosphorylation. Using direct visualization of protein–protein interaction by FRET and various in vitro and in vivo approaches we have demonstrated that CaMKII-dependent phosphorylation of KIF17 on Ser 1029 disrupts the KIF17–Mint1 association and results in the release of the transported cargo from its microtubule-based transport.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hirokawa, N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279, 519–526 (1998).
Vale, R. D. The molecular motor toolbox for intracellular transport. Cell 112, 467–480 (2003).
Hirokawa, N. & Takemura, R. Molecular motors and mechanisms of directional transport in neurons. Nature Rev. Neurosci. 6, 201–214 (2005).
Setou, M., Nakagawa, T., Seog, D. H. & Hirokawa, N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science 288, 1796–1802 (2000).
Nakagawa, T. et al. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell 103, 537–540 (2000).
Verhey, K. J. et al. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J. Cell Biol. 152, 959–970 (2001).
Setou, M. et al. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature 417, 83–87 (2002).
Nakata, T. & Hirokawa, N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J. Cell Biol. 162, 1045–1055 (2003).
Sampo, B., Kaech, S., Kunz, S. & Banker, G. Two distinct mechanisms target membrane proteins to the axonal surface. Neuron 37, 611–624 (2003).
Kikkawa, M. et al. Switch-based mechanism of kinesin motors. Nature 411, 439–445 (2001).
Okada, Y., Higuchi, H. & Hirokawa, N. Processivity of the single-headed kinesin KIF1A through biased binding to tubulin. Nature 424, 574–577 (2003).
Kamal, A. & Goldstein, L. S. B. Principles of cargo attachment to cytoplasmic motor proteins. Curr. Opin. Cell Biol. 14, 63–68 (2002).
Karcher, R. L. et al. Cell cycle regulation of myosin-V by calcium/calmodulin-dependent protein kinase II. Science 293, 1317–1320 (2001).
Allan, V. I. & Vale, R. D. Cell cycle control of microtubule-based membrane transport and tubule formation in vitro. J. Cell Biol. 113, 347–359 (1991).
Sato-Yoshitake, R., Yorifuji, H., Inagaki, M. & Hirokawa, N. The phosphorylation of kinesin regulates its binding to synaptic vesicles. J. Biol. Chem. 267, 23930–23936 (1992).
Tsai, M., Morfini, G., Szebenyi, G. & Brady, S. T. Release of kinesin from vesicles by hsc70 and regulation of fast axonal transport. Mol. Biol. Cell 11, 2161–2173 (2000).
Stagi, M., Gorlovoy, P., Larionov, S., Takahashi, K. & Neumann, H. Unloading kinesin transported cargoes from the tubulin track via the inflammatory c-Jun N-terminal kinase pathway. FASEB J. 14, 2573–2575 (2006).
Miki, H., Okada, Y. & Hirokawa, N. Analysis of the kinesin superfamily: insights into structure and function. Trends Cell Biol. 15, 467–476 (2005).
Guillaud, L., Setou, M. & Hirokawa, N. KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J. Neurosci. 23, 131–140 (2003).
Wong, R. W., Setou, M., Teng, J., Takei, Y. & Hirokawa, N. Overexpression of motor protein KIF17 enhances spatial and working memory in transgenic mice. Proc. Natl Acad. Sci. USA 99, 14500–14505 (2002).
Hollenbeck, P. J. Phosphorylation of neuronal kinesin heavy and light chains in vivo. J. Neurochem. 60, 2265–2275 (1993).
Morfini, G., Szebenyi, G., Elluru, R., Ratner, N. & Brady, S. T. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 21, 281–293 (2002).
Kennelly, P. J. & Krebs, E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J. Biol. Chem. 266, 15555–15558 (1991).
Sparks, A. B., Rider, J. E. & Kay, B. K. Mapping the specificity of SH3 domains with phage-displayed random-peptide libraries. Methods Mol. Biol. 84, 87–103 (1998).
Anderson, J. M. Cell signalling: MAGUK magic. Curr. Biol. 6, 382–384 (1996).
Shen, K. & Meyer, T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science 284, 162–166 (1999).
Long, J. F. et al. Autoinhibition of X11/Mint scaffold proteins revealed by the closed conformation of the PDZ tandem. Nature Struct. Mol. Biol. 12, 722–728 (2005).
Costa, M. C. R., Mani, F., Santoro, W. Jr, Espreafico, E. M. & Larson, R. E. Brain myosin-V, a calmodulin-carrying myosin, binds to calmodulin-dependent protein kinase II and activates its kinase activity. J. Biol. Chem. 274, 15811–15819 (1999).
Lisman, J., Schulman, H. & Cline, H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nature Rev. Neurosci. 3, 175–190 (2002).
Washbourne, P., Liu, X. B., Jones, E. G. & McAllister, A. K. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J. Neurosci. 90, 3950–3957 (2004).
Groc, L. Choquet D. AMPA and NMDA glutamate receptor trafficking: multiple roads for reaching and leaving the synapse. Cell Tissue Res. 326, 423–438 (2006).
Lau, C. G. & Zukin, R. S. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nature Rev. Neurosci. 8, 413–426 (2007).
Mok, H. et al. Association of the kinesin superfamily motor protein KIF1B(with postsynaptic density-95 (PSD-95), synapse-associated protein-97, and synaptic scaffolding molecule PSD-95/Discs Large/Zona Occludens-1 proteins. J. Neurosci. 22, 5253–5258 (2002).
Chen, P., Gu, Z., Liu, W. & Yan, Z. Glycogen synthase kinase 3 regulates N-methyl-D-aspartate receptor channel trafficking and function in cortical neurons. Mol. Pharmacol. 72, 40–51 (2007).
Forster, T. Intermolecular energy migration and fluorescence. Ann. Phys. (Leipzig) 2, 55–75 (1948).
Miyawaki, A. & Tsien, R. Y. Monitoring protein conformations and interactions by fluorescence resonance energy transfer between mutants of green fluorescent protein. Methods Enzymol. 327, 472–500 (2000).
Spiegel, R. M. et al. Measurement of molecular interactions in living cells by fluorescence resonance energy transfer between variants of the green fluorescent protein. Sci. STKE 38, l1 (2000).
Frank, R. & Overwin, H. SPOT synthesis. Epitope analysis with arrays of synthetic peptides prepared on cellulose membranes. Meth. Mol. Biol. 66, 149–169 (1996).
Boyle, W. J., Van der, Geer, P. & Hunter, T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 201, 110–149 (1991).
Acknowledgements
We thank T. Meyer (Stanford University, CA) for the GFP–CaMKII constructs, Y. Okada (Hirokawa Laboratory) for helpful discussions and S. Hiromi (Hirokawa Laboratory) for the hippocampal neuron preparations. This work was supported by a grant-in-aid for specially-promoted research from the Japanese Ministry of Education, Culture, Sports, Science and Technology to N.H.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Guillaud, L., Wong, R. & Hirokawa, N. Disruption of KIF17–Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin–cargo release. Nat Cell Biol 10, 19–29 (2008). https://doi.org/10.1038/ncb1665
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1665
This article is cited by
-
Myosin Va-dependent Transport of NMDA Receptors in Hippocampal Neurons
Neuroscience Bulletin (2024)
-
KIF17 Modulates Epileptic Seizures and Membrane Expression of the NMDA Receptor Subunit NR2B
Neuroscience Bulletin (2022)
-
Kif17 phosphorylation regulates photoreceptor outer segment turnover
BMC Cell Biology (2018)
-
Kinesin Family of Proteins Kif11 and Kif21B Act as Inhibitory Constraints of Excitatory Synaptic Transmission Through Distinct Mechanisms
Scientific Reports (2018)
-
The impact of cytoskeletal organization on the local regulation of neuronal transport
Nature Reviews Neuroscience (2017)