Abstract
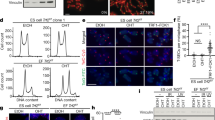
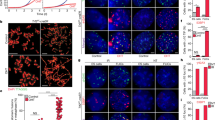
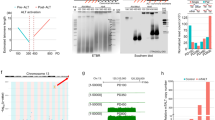
Stem cells and cancer cells maintain telomere length mostly through telomerase1,2,3. Telomerase activity is high in male germ line and stem cells, but is low or absent in mature oocytes and cleavage stage embryos, and then high again in blastocysts3. How early embryos reset telomere length remains poorly understood. Here, we show that oocytes actually have shorter telomeres than somatic cells, but their telomeres lengthen remarkably during early cleavage development. Moreover, parthenogenetically activated oocytes also lengthen their telomeres, thus the capacity to elongate telomeres must reside within oocytes themselves. Notably, telomeres also elongate in the early cleavage embryos of telomerase-null mice, demonstrating that telomerase is unlikely to be responsible for the abrupt lengthening of telomeres in these cells. Coincident with telomere lengthening, extensive telomere sister-chromatid exchange (T-SCE) and colocalization of the DNA recombination proteins Rad50 and TRF1 were observed in early cleavage embryos. Both T-SCE and DNA recombination proteins decrease in blastocyst stage embryos, whereas telomerase activity increases and telomeres elongate only slowly. We suggest that telomeres lengthen during the early cleavage cycles following fertilization through a recombination-based mechanism, and that from the blastocyst stage onwards, telomerase only maintains the telomere length established by this alternative mechanism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Allsopp, R. C. et al. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl Acad. Sci. USA 89, 10114–10118 (1992).
Kim, N. W. et al. Specific association of human telomerase activity with immortal cells and cancer. Science 266, 2011–2015 (1994).
Wright, W. E., Piatyszek, M. A., Rainey, W. E., Byrd, W. & Shay, J. W. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 18, 173–179 (1996).
Hande, M. P., Samper, E., Lansdorp, P. & Blasco, M. A. Telomere length dynamics and chromosomal instability in cells derived from telomerase null mice. J. Cell Biol. 144, 589–601 (1999).
Prowse, K. R. & Greider, C. W. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc. Natl Acad. Sci. USA 92, 4818–4822 (1995).
Poon, S. S., Martens, U. M., Ward, R. K. & Lansdorp, P. M. Telomere length measurements using digital fluorescence microscopy. Cytometry 36, 267–278 (1999).
Herrera, E. et al. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. EMBO J. 18, 2950–2960 (1999).
Hemann, M. T. & Greider, C. W. Wild-derived inbred mouse strains have short telomeres. Nucleic Acids Res. 28, 4474–4478 (2000).
Cawthon, R. M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 30, e47 (2002).
Callicott, R. J. & Womack, J. E. Real-time PCR assay for measurement of mouse telomeres. Comp. Med. 56, 17–22 (2006).
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997).
Schaetzlein, S. et al. Telomere length is reset during early mammalian embryogenesis. Proc. Natl Acad. Sci. USA 101, 8034–8038 (2004).
Herrera, E., Martinez, A. C. & Blasco, M. A. Impaired germinal center reaction in mice with short telomeres. EMBO J. 19, 472–481 (2000).
Kass-Eisler, A. & Greider, C. W. Recombination in telomere-length maintenance. Trends Biochem. Sci. 25, 200–204 (2000).
Bailey, S. M., Brenneman, M. A. & Goodwin, E. H. Frequent recombination in telomeric DNA may extend the proliferative life of telomerase-negative cells. Nucleic Acids Res. 32, 3743–3751 (2004).
Bechter, O. E., Zou, Y., Walker, W., Wright, W. E. & Shay, J. W. Telomeric recombination in mismatch repair deficient human colon cancer cells after telomerase inhibition. Cancer Res. 64, 3444–3451 (2004).
Londono-Vallejo, J. A., Der-Sarkissian, H., Cazes, L., Bacchetti, S. & Reddel, R. R. Alternative lengthening of telomeres is characterized by high rates of telomeric exchange. Cancer Res. 64, 2324–2327 (2004).
Gonzalo, S. et al. DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nature Cell Biol. 8, 416–424 (2006).
Wang, S. S. & Zakian, V. A. Telomere-telomere recombination provides an express pathway for telomere acquisition. Nature 345, 456–458 (1990).
Muntoni, A. & Reddel, R. R. The first molecular details of ALT in human tumor cells. Hum. Mol. Genet. 14, R191–R196 (2005).
Bressan, D. A., Baxter, B. K. & Petrini, J. H. The Mre11–Rad50–Xrs2 protein complex facilitates homologous recombination-based double-strand break repair in Saccharomyces cerevisiae. Mol. Cell Biol. 19, 7681–7687 (1999).
Stavropoulos, D. J. et al. The Bloom syndrome helicase BLM interacts with TRF2 in ALT cells and promotes telomeric DNA synthesis. Hum. Mol. Genet. 11, 3135–3144 (2002).
Lundblad, V. Telomere maintenance without telomerase. Oncogene 21, 522–531 (2002).
Zhu, X. D., Kuster, B., Mann, M., Petrini, J. H. & de Lange, T. Cell-cycle-regulated association of RAD50–MRE11–NBS1 with TRF2 and human telomeres. Nature Genet. 25, 347–352 (2000).
Hartsuiker, E., Vaessen, E., Carr, A. M. & Kohli, J. Fission yeast Rad50 stimulates sister chromatid recombination and links cohesion with repair. EMBO J. 20, 6660–6671 (2001).
Morgan, H. D., Santos, F., Green, K., Dean, W. & Reik, W. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 14, R47–R58 (2005).
Tanemura, K. et al. Dynamic rearrangement of telomeres during spermatogenesis in mice. Dev. Biol. 281, 196–207 (2005).
Laud, P. R. et al. Elevated telomere-telomere recombination in WRN-deficient, telomere dysfunctional cells promotes escape from senescence and engagement of the ALT pathway. Genes Dev. 19, 2560–2570 (2005).
Verdun, R. E. & Karlseder, J. The DNA damage machinery and homologous recombination pathway act consecutively to protect human telomeres. Cell 127, 709–720 (2006).
Keefe, D. L., Liu, L. & Marquard, K. Telomeres and aging-related meiotic dysfunction in women. Cell Mol. Life Sci. 64, 139–143 (2007).
Baird, D. M. et al. Telomere instability in the male germline. Hum. Mol. Genet. 15, 45–51 (2006).
Lanza, R. P. et al. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science 288, 665–669 (2000).
Wakayama, T. et al. Cloning of mice to six generations. Nature 407, 318–319 (2000).
Acknowledgements
We thank V. Zakian and J. Petrini for advice on the Rad50 and telomere recombination work, P. Lansdorp for providing TFL telomere-analysis software and advice on Q-FISH analysis, and I. Hickson for providing anti-BLM antibodies. This work was partly supported by the Women and Infants Hospital/Brown Faculty Research (D.L.K. and L.L.), National Nature Science Foundation China (L.L.), James and Esther King Biomedical Research Program (L.L.) and the MCyT, European Union and the Josef Steiner Award 2003 (M.A.B.).
Author information
Authors and Affiliations
Contributions
L.L. and D.L.K. designed the study. L.L., S.M.B., M.O., P.M., C.L., L.Z., C.W., E.C., L.S. and A.S. performed the experiments. M.A.B. provided the TRKO mice and advice. L.L. and D.L.K. wrote the paper, and S.M.B, M.A.B. and A.S. revised the manuscript.
Corresponding authors
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4, S5 and S6 (PDF 2315 kb)
Rights and permissions
About this article
Cite this article
Liu, L., Bailey, S., Okuka, M. et al. Telomere lengthening early in development. Nat Cell Biol 9, 1436–1441 (2007). https://doi.org/10.1038/ncb1664
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1664
This article is cited by
-
Chromosome ends and the theory of marginotomy: implications for reproduction
Biogerontology (2024)
-
The crosstalk between telomeres and DNA repair mechanisms: an overview to mammalian somatic cells, germ cells, and preimplantation embryos
Journal of Assisted Reproduction and Genetics (2024)
-
Telomere-based treatment strategy of cardiovascular diseases: imagination comes to reality
Genome Instability & Disease (2024)
-
Short telomeres impede germ cell specification by upregulating MAPK and TGFβ signaling
Science China Life Sciences (2023)
-
Human granulosa cells of poor ovarian responder patients display telomeres shortening
Journal of Assisted Reproduction and Genetics (2023)