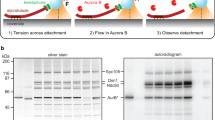
Abstract
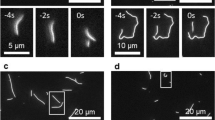
In dividing cells, kinetochores couple chromosomes to the tips of growing and shortening microtubule fibres1,2 and tension at the kinetochore–microtubule interface promotes fibre elongation3,4,5,6. Tension-dependent microtubule fibre elongation is thought to be essential for coordinating chromosome alignment and separation1,3,7,8,9,10, but the mechanism underlying this effect is unknown. Using optical tweezers, we applied tension to a model of the kinetochore–microtubule interface composed of the yeast Dam1 complex11,12,13 bound to individual dynamic microtubule tips14. Higher tension decreased the likelihood that growing tips would begin to shorten, slowed shortening, and increased the likelihood that shortening tips would resume growth. These effects are similar to the effects of tension on kinetochore-attached microtubule fibres in many cell types, suggesting that we have reconstituted a direct mechanism for microtubule-length control in mitosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Inoue, S. & Salmon, E. D. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol. Biol. Cell 6, 1619–1640 (1995).
Koshland, D. E., Mitchison, T. J. & Kirschner, M. W. Polewards chromosome movement driven by microtubule depolymerization in vitro. Nature 331, 499–504 (1988).
Nicklas, R. B. The forces that move chromosomes in mitosis. Annu. Rev. Biophys. Biophys. Chem. 17, 431–449 (1988).
Skibbens, R. V. & Salmon, E. D. Micromanipulation of chromosomes in mitotic vertebrate tissue cells: tension controls the state of kinetochore movement. Exp. Cell Res. 235, 314–324 (1997).
Khodjakov, A. & Rieder, C. L. Kinetochores moving away from their associated pole do not exert a significant pushing force on the chromosome. J. Cell Biol. 135, 315–327 (1996).
Skibbens, R. V., Rieder, C. L. & Salmon, E. D. Kinetochore motility after severing between sister centromeres using laser microsurgery: evidence that kinetochore directional instability and position is regulated by tension. J. Cell Sci. 108, 2537–2548 (1995).
Goshima, G., Wollman, R., Stuurman, N., Scholey, J. M. & Vale, R. D. Length control of the metaphase spindle. Curr. Biol. 15, 1979–1988 (2005).
Gardner, M. K. et al. Tension-dependent regulation of microtubule dynamics at kinetochores can explain metaphase congression in yeast. Mol. Biol. Cell 16, 3764–3775 (2005).
Skibbens, R. V., Skeen, V. P. & Salmon, E. D. Directional instability of kinetochore motility during chromosome congression and segregation in mitotic newt lung cells: a push-pull mechanism. J. Cell Biol. 122, 859–875 (1993).
Civelekoglu-Scholey, G., Sharp, D. J., Mogilner, A. & Scholey, J. M. Model of chromosome motility in Drosophila embryos: adaptation of a general mechanism for rapid mitosis. Biophys. J. 90, 3966–3982 (2006).
Cheeseman, I. M., Drubin, D. G. & Barnes, G. Simple centromere, complex kinetochore: linking spindle microtubules and centromeric DNA in budding yeast. J. Cell Biol. 157, 199–203 (2002).
Miranda, J. J., De Wulf, P., Sorger, P. K. & Harrison, S. C. The yeast DASH complex forms closed rings on microtubules. Nature Struct. Mol. Biol. 12, 138–143 (2005).
Westermann, S. et al. Formation of a dynamic kinetochore- microtubule interface through assembly of the Dam1 ring complex. Mol. Cell 17, 277–290 (2005).
Asbury, C. L., Gestaut, D. R., Powers, A. F., Franck, A. D. & Davis, T. N. The Dam1 kinetochore complex harnesses microtubule dynamics to produce force and movement. Proc. Natl Acad. Sci. USA 103, 9873–9878 (2006).
Inoue, S. & Ritter, H., Jr. Dynamics of mitotic spindle organization and function. Soc. Gen. Physiol. Ser. 30, 3–30 (1975).
Nicklas, R. B. Measurements of the force produced by the mitotic spindle in anaphase. J. Cell Biol. 97, 542–548 (1983).
Walker, R. A. et al. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J. Cell Biol. 107, 1437–1448 (1988).
Maddox, P., Straight, A., Coughlin, P., Mitchison, T. J. & Salmon, E. D. Direct observation of microtubule dynamics at kinetochores in Xenopus extract spindles: implications for spindle mechanics. J. Cell Biol. 162, 377–382 (2003).
Andrews, P. D. et al. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell 6, 253–268 (2004).
Cimini, D., Wan, X., Hirel, C. B. & Salmon, E. D. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr. Biol. 16, 1711–1718 (2006).
Pinsky, B. A., Kung, C., Shokat, K. M. & Biggins, S. The Ipl1–Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nature Cell Biol. 8, 78–83 (2006).
Dogterom, M. & Yurke, B. Measurement of the force-velocity relation for growing microtubules. Science 278, 856–860 (1997).
Janson, M. E., de Dood, M. E. & Dogterom, M. Dynamic instability of microtubules is regulated by force. J. Cell Biol. 161, 1029–1034 (2003).
Grishchuk, E. L., Molodtsov, M. I., Ataullakhanov, F. I. & McIntosh, J. R. Force production by disassembling microtubules. Nature 438, 384–388 (2005).
Waters, J. C., Skibbens, R. V. & Salmon, E. D. Oscillating mitotic newt lung cell kinetochores are, on average, under tension and rarely push. J. Cell Sci. 109, 2823–31 (1996).
Westermann, S. et al. The Dam1 kinetochore ring complex moves processively on depolymerizing microtubule ends. Nature 440, 565–569 (2006).
Belmont, L. D., Hyman, A. A., Sawin, K. E. & Mitchison, T. J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell 62, 579–589 (1990).
Kinoshita, K., Arnal, I., Desai, A., Drechsel, D. N. & Hyman, A. A. Reconstitution of physiological microtubule dynamics using purified components. Science 294, 1340–1343 (2001).
Verde, F., Dogterom, M., Stelzer, E., Karsenti, E. & Leibler, S. Control of microtubule dynamics and length by cyclin A- and cyclin B-dependent kinases in Xenopus egg extracts. J. Cell Biol. 118, 1097–1108 (1992).
Rieder, C. L., Davison, E. A., Jensen, L. C., Cassimeris, L. & Salmon, E. D. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster and half-spindle. J. Cell Biol. 103, 581–591 (1986).
Joglekar, A. P. & Hunt, A. J. A simple, mechanistic model for directional instability during mitotic chromosome movements. Biophys J. 83, 42–58 (2002).
Pearson, C. G. et al. Stable kinetochore-microtubule attachment constrains centromere positioning in metaphase. Curr. Biol. 14, 1962–1967 (2004).
Janson, M. E. & Dogterom, M. Scaling of microtubule force-velocity curves obtained at different tubulin concentrations. Phys. Rev. Lett. 92, 248101 (2004).
Mandelkow, E. M., Mandelkow, E. & Milligan, R. A. Microtubule dynamics and microtubule caps: a time-resolved cryo-electron microscopy study. J. Cell Biol. 114, 977–991 (1991).
Acknowledgements
We thank J. J. Miranda and S. C. Harrison (Harvard Medical School) for providing the expression plasmid for the Dam1 complex, and B. Graczyk for electron microscopy sample preparation. This work was supported by a Searle Scholar Award (to C.L.A.), and by grants from the National Institutes of Health (to T.N.D. and C.L.A.). A.F.P. was supported by a National Institutes of Health (NIH) training grant, T32 GM07270.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figure S1 and Supplementary movie legends (PDF 255 kb)
Rights and permissions
About this article
Cite this article
Franck, A., Powers, A., Gestaut, D. et al. Tension applied through the Dam1 complex promotes microtubule elongation providing a direct mechanism for length control in mitosis. Nat Cell Biol 9, 832–837 (2007). https://doi.org/10.1038/ncb1609
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1609
This article is cited by
-
Probing stress-regulated ordering of the plant cortical microtubule array via a computational approach
BMC Plant Biology (2023)
-
Biomechanical, biophysical and biochemical modulators of cytoskeletal remodelling and emergent stem cell lineage commitment
Communications Biology (2023)
-
Mechanisms underlying spindle assembly and robustness
Nature Reviews Molecular Cell Biology (2023)
-
Measuring and modeling forces generated by microtubules
Biophysical Reviews (2023)
-
Regulation of microtubule dynamics, mechanics and function through the growing tip
Nature Reviews Molecular Cell Biology (2021)