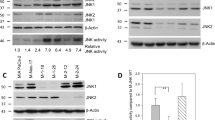
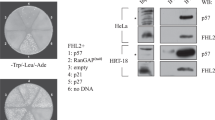
Abstract
The cause or consequence of overexpression of p73 (refs 1, 2), the structural and functional homologue of the tumour-suppressor gene product p53 (refs 3, 4), in human cancers is poorly understood. Here, we report a role for p73 in supporting cellular growth through the upregulation of AP-1 transcriptional activity. p73 suppresses growth when overexpressed alone, but synergises with the proto-oncogene c-Jun to promote cellular survival. Conversely, silencing of p73 expression compromises cellular proliferation. Molecular analysis revealed that expression of the AP-1 target-gene product cyclinD1 (ref. 5) is reduced concomitant with p73, but not p53, silencing. Moreover, cyclinD1 was induced by p73 expression in a c-Jun-dependent manner, and was required for p73-mediated cell survival. Furthermore, c-Jun-dependent AP-1 transcriptional activity was augmented by p73 and, consistently, induction of endogenous AP-1 target genes was compromised in the absence of p73. Chromatin immunoprecipitation and electrophoretic mobility shift analysis indicated that p73 enhanced the binding of phosphorylated c-Jun and Fra-1, another AP-1 family member, to AP-1 consensus DNA sequences, by regulating c-Jun phosphorylation and Fra-1 expression. Collectively, our data demonstrates a novel and unexpected role of p73 in augmenting AP-1 transcriptional activity through which it supports cellular growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zaika, A. I., Kovalev, S., Marchenko, N. D. & Moll, U. M. Overexpression of the wild type p73 gene in breast cancer tissues and cell lines. Cancer Res. 59, 3257–3263 (1999).
Concin, N. et al. Transdominant DeltaTAp73 isoforms are frequently up-regulated in ovarian cancer. Evidence for their role as epigenetic p53 inhibitors in vivo. Cancer Res. 64, 2449–2460 (2004).
Yang, A., Kaghad, M., Caput, D. & McKeon, F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 18, 90–95 (2002).
Melino, G., De Laurenzi, V. & Vousden, K. H. p73: Friend or foe in tumorigenesis. Nature Rev. Cancer 2, 605–615 (2002).
Herber, B., Truss, M., Beato, M. & Muller, R. Inducible regulatory elements in the human cyclin D1 promoter. Oncogene 9, 1295–1304 (1994).
Toh, W. H., Siddique, M. M., Boominathan, L, Lin, K. W. & Sabapathy, K. c-Jun regulates the stability and activity of the p53 homologue, p73. J. Biol. Chem. 279, 44713–44722 (2004).
Fu, M., Wang, C., Li, Z., Sakamaki, T. Pestell, R. G. Cyclin D1: normal and abnormal functions. Endocrinology 145, 5439–5447 (2004).
el Deiry, W. S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Blint, E., Phillips, A. C., Kozlov, S., Stewart, C. L. & Vousden, K. H. Induction of p57 (KIP2) expression by p73β. Proc. Natl Acad. Sci. USA 99, 3529–3534 (2002).
Pozniak, C. D. et al. An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science 289, 304–306 (2000).
Agami, R., Blandino, G., Oren, M. & Shaul, Y. Interaction of c-Abl and p73α and their collaboration to induce apoptosis. Nature 399, 809–813 (1999).
Gaiddon, C. et al. Cyclin-dependent kinases phosphorylate p73 at threonine 86 in a cell cycle-dependent manner and negatively regulate p73. J. Biol. Chem. 278, 27421–27431 (2003).
Fulco, M. et al. p73 is regulated by phosphorylation at the G2/M transition. J. Biol. Chem. 278, 49196–49202 (2003).
Mantovani, F. et al. Pin1 links the activities of c-Abl and p300 in regulating p73 function. Mol. Cell 14, 625–636 (2004).
Barila, D. et al. A nuclear tyrosine phosphorylation circuit: c-Jun as an activator and substrate of c-Abl and JNK. EMBO J. 19, 273–281 (2000).
Angel, P. et al. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell 49, 729–739 (1987).
Matsuo, K. et al. Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nature Genet. 24, 184–187 (2000).
Cawley, S. et al. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell 116, 499–509 (2004).
Han, Z. et al. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Invest. 108, 73–81 (2001).
Behrens, A. et al. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nature Genet. 21, 326–329 (1999).
Zhu, J., Jiang, J., Zhou, W. & Chen, X. The potential tumor suppressor p73 differentially regulates cellular p53 target genes. Cancer Res. 58, 5061–5065 (1998).
Toh, W. H., Kyo, S. & Sabapathy, K. Relief of p53-mediated telomerase suppression by p73. J. Biol. Chem. 280, 17329–17338 (2005).
Yang, A. et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 404, 99–103 (2000).
Jacks, T. et al. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4, 1–7 (1994).
Harvey, M. et al. In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene 8, 2457–2467 (1993).
Hilberg, F. et al. c-jun is essential for normal mouse development and hepatogenesis. Nature 365, 179–181 (1993).
Eferl, R. et al. Functions of c-Jun in liver and heart development. J. Cell Biol. 145, 1049–1061 (1999).
Raivich, G. et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron 43, 57–67 (2004).
Sabapathy, K., Hochedlinger, K., Nam, S. Y., Bauer, A., Karin, M. & Wagner E. F. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol. Cell 15, 713–725 (2004).
Passegue, E. & Wagner, E. F. JunB suppresses cell proliferation by transcriptional activation of p16 (INK4a) expression. EMBO J. 19, 2969–2679 (2000).
Acknowledgements
We thank K. W. Lin for technical assistance. We are grateful to A. Behrens, D. Bohmann, G. Blandino, M. Levrero, G. Melino, G. Del Sal and E. Passegue for the various plasmids, and to J. Wang, P. Sicinski and A. Behrens for the p73−/− fibroblasts, the cyclinD1+/+ and cyclinD1−/− cells and the junAA cells, respectively. F.V. is a visiting Scientist from Institute Cytology, St.Petersburg. This study was supported by grants from the National Medical Research Council and Biomedical Research Council of Singapore to K.S.
Author information
Authors and Affiliations
Contributions
F.V. performed most of the experimental work presented in the paper. T.W.H. performed: the work on stable p73 knockdown cells; the Fra-1 siRNA experiments; the reporter assay to establish the role of c-JunY170F in the absence of c-Jun; the role of the Pin-1 and CHK2 p73 mutants, and using the junAA cells; and determined the status of phosphorylated c-Jun in p53- and p73-null cells. I.D. was involved in: the quantification of c-Jun phophorylation on p73 induction; the JNK inhibitor experiments; and the generation and characterization of the JNK-null cells in which p73 expression was inducible. W.Q. and N.H.H. performed the ChIP experiments. L.B. performed initial luciferase reporter experiments when attached to the laboratory for a brief period. K.V. provided the SAOS–p73β inducible cells and advised on the initial part of the work. K.S. was responsible for the overall project, in particular, coming up with the ideas and planning the experimental strategies, writing the paper and securing funding for the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4 and Supplementary Table S1 (PDF 346 kb)
Rights and permissions
About this article
Cite this article
Vikhanskaya, F., Toh, W., Dulloo, I. et al. p73 supports cellular growth through c-Jun-dependent AP-1 transactivation. Nat Cell Biol 9, 698–706 (2007). https://doi.org/10.1038/ncb1598
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1598
This article is cited by
-
BRD4 drives esophageal squamous cell carcinoma growth by promoting RCC2 expression
Oncogene (2022)
-
The p53 family member p73 in the regulation of cell stress response
Biology Direct (2021)
-
Cyclin D1 is a mediator of gastrointestinal stromal tumor KIT-independence
Oncogene (2019)
-
Long noncoding RNA LINC01111 suppresses pancreatic cancer aggressiveness by regulating DUSP1 expression via microRNA-3924
Cell Death & Disease (2019)
-
Transcriptional regulation of P63 on the apoptosis of male germ cells and three stages of spermatogenesis in mice
Cell Death & Disease (2018)