Abstract
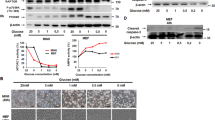
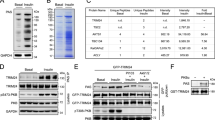
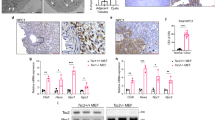
Insulin stimulates protein synthesis and cell growth by activation of the protein kinases Akt (also known as protein kinase B, PKB) and mammalian target of rapamycin (mTOR). It was reported that Akt activates mTOR by phosphorylation and inhibition of tuberous sclerosis complex 2 (TSC2)1,2,3,4. However, in recent studies the physiological requirement of Akt phosphorylation of TSC2 for mTOR activation has been questioned5,6. Here, we identify PRAS40 (proline-rich Akt/PKB substrate 40 kDa) as a novel mTOR binding partner that mediates Akt signals to mTOR. PRAS40 binds the mTOR kinase domain and its interaction with mTOR is induced under conditions that inhibit mTOR signalling, such as nutrient or serum deprivation or mitochondrial metabolic inhibition. Binding of PRAS40 inhibits mTOR activity and suppresses constitutive activation of mTOR in cells lacking TSC2. PRAS40 silencing inactivates insulin-receptor substrate-1 (IRS-1) and Akt, and uncouples the response of mTOR to Akt signals. Furthermore, PRAS40 phosphorylation by Akt and association with 14-3-3, a cytosolic anchor protein, are crucial for insulin to stimulate mTOR. These findings identify PRAS40 as an important regulator of insulin sensitivity of the Akt–mTOR pathway and a potential target for the treatment of cancers, insulin resistance and hamartoma syndromes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Inoki, K., Li, Y., Zhu, T., Wu, J. & Guan, K. L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nature Cell Biol. 4, 648–657 (2002).
Manning, B. D., Tee, A. R., Logsdon, M. N., Blenis, J. & Cantley, L. C. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinsitide 3-kinase/Akt pathway. Mol. Cell 10, 151–162 (2002).
Zhang, Y. et al. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nature Cell Biol. 5, 578–581 (2003).
Long, X., Lin, Y., Ortiz-Vega, S., Yonezawa, K. & Avruch, J. Rheb binds and regulates the mTOR kinase. Curr. Biol. 15, 702–713 (2005).
Dong, J. & Pan, D. J. Tsc2 is not a critical target of Akt during normal Drosophila development. Genes Dev. 18, 2479–2484 (2004).
Hahn-Windgassen, A. et al. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J. Biol. Chem. 280, 32081–32089 (2005).
Brunn, G. J. et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277, 99–101 (1997).
Dennis, P. B. et al. Mammalian TOR, a homeostatic ATP sensor. Science 294, 1102–1105 (2001).
Shimji, A. F., Nghiem, P. & Schreiber, S. L. Integration of growth factor and nutrient signaling: implications for cancer biology. Molecular Cell 12, 271–280 (2003).
Gao, X. et al. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nature Cell Biol. 4, 699–704 (2002).
Corradetti, M. N. Inoki, K., Bardeesy, N., Depinho, R. A. & Guan, K. L. Regulation of the TSC pathway by LKB1: evidence of a molecular link between tuberous sclerosis complex and Peutz-Jeghers syndrome. Genes Dev. 18, 1533–1538 (2004).
Um, S. H. et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431, 200–205 (2004).
Kenerson, H., Dundon, T. A. & Yeung, R. S. Effects of rapamycin in the Eker rat model of tuberous sclerosis complex. Pediatr Res. 57, 67–75 (2005).
Vellai, T. et al. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426, 620 (2003).
Loewith, R. et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10, 457–468 (2002).
Kim, D.-H. et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 (2002).
Kim, D.-H. et al. GβL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol. Cell 11, 895–904 (2003).
Hara, K. et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177–189 (2002).
Sarbassov, D. D. et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 (2004).
Sarbassov, D. D., Guertin, D. A., Ali, S. M. & Sabatini, D. M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 (2005).
Jacinto, E. et al. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nature Cell Biol. 6, 1122–1128 (2004).
Eng, J., McCormack, A. L. & Yates, J. R. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Mass Spectrom. 5, 976–989 (1994).
Keller, A., Nesvizhskii, A. I., Kolker, E. & Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 (2002).
Frias, M. A. et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 16, 1–6 (2006).
Jacinto, E. et al. Sin1/Mip1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 126, 1–13 (2006).
Kovacina, K. S. et al. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J. Biol. Chem. 278, 10189–10194 (2003).
Singh, A., Chan, J., Chern, J. J. & Choi, K.-W. Genetic interaction of lobe with its modifiers in dorsoventral patterning and growth of the Drosophila eye. Genetics 171, 169–183 (2005).
Tzatsos, A. & Kandror, K. V. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol. Cell. Biol. 26, 63–76 (2006).
Huang, B. & Porter, G. Expression of proline-rich akt-substrate PRAS40 in cell survival pathway and carcinogenesis. Acta. Pharmacol. Sin. 26, 1253–1258 (2005).
Saito, A. et al. Neuroprotective role of a proline-rich Akt substrate in apoptotic neuronal cell death after stroke: relationships with nerve growth factor. J. Neurosci. 24, 1584–1593 (2004).
Acknowledgements
We thank: R. Roth for PRAS40 cDNA; A. Shaw for 14-3-3 plasmid; R. Yeung for Tsc2 cells; Y.-J. Kim for database search; H. Towle and T. Neufeld for comments on the manuscript; the Mass Spectrometry and Proteomics Center at the University of Minnesota for the mass spectrometry instrumentation; the University of Minnesota Supercomputing Institute for the Sequest cluster. This study was supported by the Tuberous Sclerosis Alliance, the Minnesota Medical Foundation, the Department of Defense TS050039, and the National Institutes of Health (grant DK072004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4, Supplementary tables S1 and S2 (PDF 1877 kb)
Rights and permissions
About this article
Cite this article
Haar, E., Lee, Si., Bandhakavi, S. et al. Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9, 316–323 (2007). https://doi.org/10.1038/ncb1547
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1547
This article is cited by
-
Pathogenic mutations of human phosphorylation sites affect protein–protein interactions
Nature Communications (2024)
-
Targeting dysregulated phago-/auto-lysosomes in Sertoli cells to ameliorate late-onset hypogonadism
Nature Aging (2024)
-
Exploring the potential of Ziziphus nummularia and luteolin-7-O-glucoside as tubulin inhibitors in cancer therapy and survival
Scientific Reports (2024)
-
mTORC1 in energy expenditure: consequences for obesity
Nature Reviews Endocrinology (2024)
-
Expression of mTOR in normal and pathological conditions
Molecular Cancer (2023)