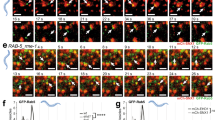
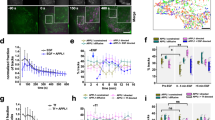
Abstract
The early endosome is organised into domains to ensure the separation of cargo1,2. Activated mitogenic receptors, such as epidermal growth factor (EGF) receptor, are concentrated into vacuoles enriched for the small GTPase Rab53,4, which progressively exclude nutrient receptors, such as transferrin receptor, into neighbouring tubules4,5,6,7. These vacuoles become enlarged, increase their content of intralumenal vesicles as EGF receptor is sorted from the limiting membrane, and eventually mature to late endosomes8. Maturation is governed by the loss of Rab5 and is accompanied by the movement of endosomes along microtubules towards the cell centre9. Here, we show that EGF relocates to the cell centre in a dynein-dependent fashion, concomitant with the sorting away of transferrin receptor, although it remains in Rab5-positive early endosomes. When dynein function is acutely disrupted, efficient recycling of transferrin from EGF-containing endosomes is retarded, loss of Rab5 is slowed and endosome enlargement is reduced.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gruenberg, J. The endocytic pathway: a mosaic of domains. Nature Rev. Mol. Cell. Biol. 2, 721–730 (2001).
Zerial, M. & McBride, H. Rab proteins as membrane organizers. Nature Rev. Mol. Cell. Biol. 2, 107–117 (2001).
Felder, S. et al. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell 61, 623–624 (1990).
Sönnichsen, B., De Renzis, S., Nielsen, E., Rietdorf, J. & Zerial, M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J. Cell Biol. 149, 901–914 (2000).
Sheff, D. R., Daro, E. A., Hull, M. & Mellman, I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145, 123–129 (1999).
van der Sluijs, P. et al. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 70, 729–740 (1992).
Ullrich, O., Reinsch, S., Urbé, S., Zerial, M. & Parton, R. Rab11 regulates recycling through the pericentriolar recycling endosome. J. Cell Biol. 135, 913–924 (1996).
Futter, C. E., Pearse, A., Hewlett, L. J. & Hopkins, C. R. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse with lysosomes. J. Cell Biol. 132, 1011–1023 (1996).
Rink, J., Ghigo, E., Kalaidzidis, Y. & Zerial, M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 122, 735–749 (2005).
Allan, V. J. & Schroer, T. A. Membrane motors. Curr. Opin. Cell Biol. 11, 476–482 (1999).
Valetti, C. et al. Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol. Biol. Cell 10, 4107–4120 (1999).
Jordens, I. et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 11, 1680–1685 (2001).
Lebrand, C. et al. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 21, 1289–1300 (2002).
Echeverri, C. J., Paschal, B. M., Vaughan, K. T. & Vallee, R. B. Molecular characterisation of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organisation during mitosis. J. Cell Biol. 132, 617–633 (1996).
Burkhardt, J., Echeverri, C., Nilsson, T. & Vallee, R. Overexpression of the Dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139, 469–484 (1997).
Deacon, S. W. et al. Dynactin is required for bidirectional organelle transport. J. Cell Biol. 160, 297–301 (2003).
Blangy, A., Arnaud, L. & Nigg, E. A. Phosphorylation by p34cdc2 protein kinase regulates binding of the kinesin-related motor HsEg5 to the dynactin subunit p150glued. J. Biol. Chem. 272, 19418–19424 (1997).
Quintyne, N. J. et al. Dynactin is required for microtubule anchoring at centrosomes. J. Cell Biol. 147, 321–334 (1999).
King, S., Brown, C., Maier, K., Quintyne, N. & Schroer, T. Analysis of the dynein-dynactin interaction in vitro and in vivo. Mol. Biol. Cell 14, 5089–5097 (2003).
Nielsen, E., Severin, F., Backer, J., Hyman, A. & Zerial, M. Rab5 regulates motility of early endosomes on microtubules. Nature Cell Biol. 1, 376–382 (1999).
Hoepfner, S. et al. Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell 121, 437–450 (2005).
Mellman, I. Endocytosis and molecular sorting. Ann. Rev. Cell Dev. Biol. 12, 575–625 (1996).
Lakadamyali, M., Rust, M. J. & Zhuang, X. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell 124, 997–1009 (2006).
Stoorvogel, W., Strous, G. J., Geuze, H. J., Oorschot, V. & Schwartz, A. L. Late endosomes derive from early endosomes by maturation. Cell 65, 417–427 (1991).
Hopkins, C. R., Gibson, A., Shipman, M. & Miller, K. Movement of internalized ligand-receptor complexes along a continuous endosomal reticulum. Nature 346, 335–340 (1990).
White, I. J., Bailey, L. M., Aghakhani, M. R., Moss, S. E. & Futter, C. E. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 25, 1–12 (2006).
Aniento, F., Emans, N., Griffiths, G. & Gruenberg, J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J. Cell Biol. 123, 1373–1387 (1993).
Bananis, E., Murray, J. W., Stockert, R. J., Satir, P. & Wolkoff, A. W. Regulation of early endocytic vesicle motility and fission in a reconstituted system. J. Cell Sci. 116, 2749–2761 (2003).
Bananis, E., Murray, J. W., Stockert, R. J., Satir, P. & Wolkoff, A. W. Microtubule and motor-dependent endocytic vesicle sorting in vitro. J. Cell Biol. 151, 179–186 (2000).
Miller, K., Beardmore, J., Kanety, H., Schlessinger, J. & Hopkins, C. Localization of the epidermal growth factor (EGF) receptor within the endosome of EGF-stimulated epidermoid carcinoma (A431) cells. J. Cell Biol. 102, 500–509 (1986).
Yamashiro, D. J. & Maxfield, F. R. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell 37, 789–800 (1984).
Hopkins, C. R., Gibson, A., Shipman, M., Strickland, D. K. & Trowbridge, I. S. In migrating fibroblasts recycling receptors are concentrated in narrow tubules in the pericentriolar area and then routed to the plasma membrane of the leading lamella. J. Cell Biol. 125, 1265–1274 (1994).
De Brabander, M., Nuydens, R., Geerts, H. & Hopkins, C. R. Dynamic behavior of the transferrin receptor followed in living epidermoid carcinoma (A431) cells with nanovid microscopy. Cell Motil. Cytoskeleton 9, 30–47 (1988).
Sheff, D., Pelletier, L., O'Connell, C. B., Warren, G. & Mellman, I. Transferrin receptor recycling in the absence of perinuclear recycling endosomes. J. Cell Biol. 156, 797–804 (2002).
Mayhew, T. M., Lucocq, J. M. & Griffiths, G. Relative labelling index: a novel stereological approach to test for non-random immunogold labelling of organelles and membranes on transmission electron microscopy thin sections. J. Microsc. 205, 153–164 (2002).
Acknowledgements
We thank our colleagues for their generous gifts of reagents. This work is supported by the Medical Research Council (Grants G9722026, G0001128) and Biotechnology and Biological Sciences Research Council (Grant BB/C512929/1). O.J.D. was supported by a BBSRC research studentship.
Author information
Authors and Affiliations
Contributions
All authors contributed to experimental work and data analysis. O.J.D., V.J.A. and P.G.W. contributed to project planning.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3, S4 and Supplementary Movie Legends (PDF 720 kb)
Supplementary Information
Supplementary Movie 1 (MOV 4148 kb)
Supplementary Information
Supplementary Movie 2 (MOV 4506 kb)
Supplementary Information
Supplementary Movie 3 (MOV 6459 kb)
Rights and permissions
About this article
Cite this article
Driskell, O., Mironov, A., Allan, V. et al. Dynein is required for receptor sorting and the morphogenesis of early endosomes. Nat Cell Biol 9, 113–120 (2007). https://doi.org/10.1038/ncb1525
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1525
This article is cited by
-
Deterministic early endosomal maturations emerge from a stochastic trigger-and-convert mechanism
Nature Communications (2023)
-
Ensemble heterogeneity mimics ageing for endosomal dynamics within eukaryotic cells
Scientific Reports (2023)
-
Rapid whole cell imaging reveals a calcium-APPL1-dynein nexus that regulates cohort trafficking of stimulated EGF receptors
Communications Biology (2021)
-
Tubulin carboxypeptidase activity of vasohibin-1 inhibits angiogenesis by interfering with endocytosis and trafficking of pro-angiogenic factor receptors
Angiogenesis (2021)
-
Flotillins promote T cell receptor sorting through a fast Rab5–Rab11 endocytic recycling axis
Nature Communications (2019)