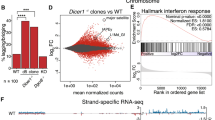
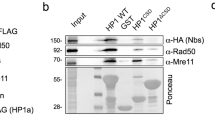
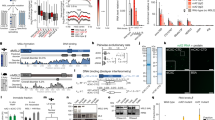
Abstract
Investigations aimed at identifying regulators of nuclear architecture in Drosophila demonstrated that cells lacking H3K9 methylation and RNA interference (RNAi) pathway components displayed disorganized nucleoli, ribosomal DNA (rDNA) and satellite DNAs. The levels of H3K9 dimethylation (H3K9me2) in chromatin associated with repeated DNAs decreased dramatically in Su(var)3-9 and dcr-2 (dicer-2) mutant tissues compared with wild type. We also observed a substantial increase in extrachromosomal circular (ecc) repeated DNAs in mutant tissues. The disorganized nucleolus phenotype depends on the presence of Ligase 4 and ecc DNA formation is not induced by removal of cohesin. We conclude that the structural integrity and organization of repeated DNAs and nucleoli are regulated by the H3K9 methylation and RNAi pathways, and other regulators of heterochromatin-mediated silencing. In addition, repeated DNA stability involves suppression of non-homologous end joining (NHEJ) or other recombination pathways. These results suggest a mechanism for how local chromatin structure can regulate genome stability, and the organization of chromosomal elements and nuclear organelles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Francastel, C., Schubeler, D., Martin, D. I. & Groudine, M. Nuclear compartmentalization and gene activity. Nature Rev. Mol. Cell Biol. 1, 137–143 (2000).
Cremer, T. & Cremer, C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nature Rev. Genet. 2, 292–301 (2001).
John, B. The biology of heterochromatin. in Heterochromatin: Molecular and Structural Aspects (ed. Verma, R. S.) 1–147 (Cambridge University Press, Cambridge, 1988).
Martin, C. & Zhang, Y. The diverse functions of histone lysine methylation. Nature Rev. Mol. Cell Biol. 6, 838–849 (2005).
Jenuwein, T. & Allis, C. D. Translating the histone code. Science 293, 1074–1080 (2001).
Muller, H. J. Types of visible variations induced by X-rays in Drosophila. J. Genet. 22, 299–334 (1930).
Schotta, G., Ebert, A., Dorn, R. & Reuter, G. Position-effect variegation and the genetic dissection of chromatin regulation in Drosophila. Semin. Cell Dev. Biol. 14, 67–75 (2003).
Pal-Bhadra, M. et al. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science 303, 669–672 (2004).
Grewal, S. I. & Moazed, D. Heterochromatin and epigenetic control of gene expression. Science 301, 798–802 (2003).
Karpen, G. H., Schaefer, J. E. & Laird, C. D. A Drosophila rRNA gene located in euchromatin is active in transcription and nucleolus formation. Genes Dev. 2, 1745–1763 (1988).
Santoro, R., Li, J. & Grummt, I. The nucleolar remodeling complex NoRC mediates heterochromatin formation and silencing of ribosomal gene transcription. Nature Genet. 32, 393–396 (2002).
Pikaard, C. S. The epigenetics of nucleolar dominance. Trends Genet. 16, 495–500 (2000).
Hawley, R. S. & Marcus, C. H. Recombinational controls of rDNA redundancy in Drosophila. Annu. Rev. Genet. 23, 87–120 (1989).
Blander, G. & Guarente, L. The Sir2 family of protein deacetylases. Annu. Rev. Biochem. 73, 417–435 (2004).
Tollervey, D., Lehtonen, H., Jansen, R., Kern, H. & Hurt, E. C. Temperature-sensitive mutations demonstrate roles for yeast fibrillarin in pre-rRNA processing, pre-rRNA methylation, and ribosome assembly. Cell 72, 443–457 (1993).
Spradling, A. & Orr-Weaver, T. Regulation of DNA replication during Drosophila development. Annu. Rev. Genet. 21, 373–403 (1987).
Schotta, G. et al. Central role of Drosophila SU(VAR)3–9 in histone H3K9 methylation and heterochromatic gene silencing. EMBO J. 21, 1121–1131 (2002).
Hirt, B. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26, 365–369 (1967).
Pont, G., Degroote, F. & Picard, G. Some extrachromosomal circular DNAs from Drosophila embryos are homologous to tandemly repeated genes. J. Mol. Biol. 195, 447–451 (1987).
Aravin, A. A. et al. The small RNA profile during Drosophila melanogaster development. Dev. Cell 5, 337–350 (2003).
Lin, Y. H. & Keil, R. L. Mutations affecting RNA polymerase I-stimulated exchange and rDNA recombination in yeast. Genetics 127, 31–38 (1991).
Cohen, S., Yacobi, K. & Segal, D. Extrachromosomal circular DNA of tandemly repeated genomic sequences in Drosophila. Genome Res 13, 1133–1145 (2003).
Cohen, Z., Bacharach, E. & Lavi, S. Mouse major satellite DNA is prone to eccDNA formation via DNA Ligase IV-dependent pathway. Oncogene 25, 4515–4524 (2006).
Kobayashi, T. & Ganley, A. R. Recombination regulation by transcription-induced cohesin dissociation in rDNA repeats. Science 309, 1581–1584 (2005).
Nonaka, N. et al. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nature Cell Biol. 4, 89–93 (2002).
Losada, A., Hirano, M. & Hirano, T. Identification of Xenopus SMC protein complexes required for sister chromatid†cohesion. Genes Dev. 12, 1986–1997 (1998).
Vakoc, C. R., Mandat, S. A., Olenchock, B. A. & Blobel, G. A. Histone H3 lysine 9 methylation and HP1γ are associated with transcription elongation through mammalian chromatin. Mol Cell 19, 381–391 (2005).
Sullivan, B. A. & Karpen, G. H. Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nature Struct. Mol. Biol. 11, 1076–1083 (2004).
Jackson, P. K. A new RING for SUMO: wrestling transcriptional responses into nuclear bodies with PIAS family E3 SUMO ligases. Genes Dev. 15, 3053–3058 (2001).
Hari, K. L., Cook, K. R. & Karpen, G. H. The Drosophila Su(var)2–10 locus encodes a member of the PIAS protein family and regulates chromosome structure and function. Genes Dev. 15, 1334–1348 (2001).
Schotta, G. et al. A silencing pathway to induce H3K9 and H4K20 trimethylation at constitutive heterochromatin. Genes Dev. 18, 1251–1262 (2004).
Lee, Y. S. et al. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 117, 69–81 (2004).
Cam, H. P. et al. Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nature Genet. 37, 809–819 (2005).
Peters, A. H. et al. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell 107, 323–337 (2001).
McKee, B. D. & Karpen, G. H. Drosophila ribosomal RNA genes function as an X–Y pairing site during male meiosis. Cell 61, 61–72 (1990).
Karpen, G. H., Le, M. H. & Le, H. Centric heterochromatin and the efficiency of achiasmate disjunction in Drosophila female meiosis. Science 273, 118–122 (1996).
Dernburg, A. F., Sedat, J. W. & Hawley, R. S. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86, 135–146 (1996).
Cartwright, I. L. et al. Analysis of Drosophila chromatin structure in vivo. Methods Enzymol. 304, 462–496 (1999).
Critchlow, S. E., Bowater, R. P. & Jackson, S. P. Mammalian DNA double-strand break repair protein XRCC4 interacts with DNA ligase IV. Curr. Biol. 7, 588–598 (1997).
Glaser, R. L., Karpen, G. H. & Spradling, A. C. Replication forks are not found in a Drosophila minichromosome demonstrating a gradient of polytenization. Chromosoma 102, 15–19 (1992).
Ivessa, A. S., Zhou, J. Q. & Zakian, V. A. The Saccharomyces Pif1p DNA helicase and the highly related Rrm3p have opposite effects on replication fork progression in ribosomal DNA. Cell 100, 479–489 (2000).
Westphal, T. & Reuter, G. Recombinogenic effects of suppressors of position-effect variegation in Drosophila. Genetics 160, 609–621 (2002).
Kaeberlein, M., McVey, M. & Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13, 2570–2580 (1999).
Osipovich, O. et al. Targeted inhibition of V(D)J recombination by a histone methyltransferase. Nature Immunol. 5, 309–316 (2004).
Peters, A. H. F. M. et al. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol. Cell 12, 1577–1589 (2003).
Dorsett, D. et al. Effects of sister chromatid cohesion proteins on cut gene expression during wing development in Drosophila. Development 132, 4743–4753 (2005).
Austin, R. J., Orr-Weaver, T. L. & Bell, S. P. Drosophila ORC specifically binds to ACE3, an origin of DNA replication control element. Genes Dev. 13, 2639–2649 (1999).
Kuschak, T. I., Kuschak, B. C., Smith, G. M., Wright, J. A. & Mai, S. Isolation of extrachromosomal elements by histone immunoprecipitation. Biotechniques 30, 1064–1068, 1070–1072 (2001).
Acknowledgements
The authors thank: J. Birchler (for hlsΔ215), R. Carthew (for DCR2L811fsx), D. Dorsett (for SMC1 antibody), S. Hawley (for smc1exc461), F. Gao (for Ago251B), T. Jenuwein (for H3K9me2 antibody), K. M. Pollard (for fibrillarin antibody), G. Reuter (for Su(var)3-9 alleles 6 and 17) and J. Rine (for dSir217); S. Weiss for experimental contributions; A. Dernburg for help with FISH experiments; and A. Blanco (formerly Metamorph) for assistance with volumetric analysis. We thank N. Bhalla, A. Dernburg, S. Erhart, P. Heun, B. Mellone, A. Minoda and W. Zhang for helpful discussions and critical comments on the manuscript. This work was supported by a National Institutes of Health (NIH) grant, R01GM061169.
Author information
Authors and Affiliations
Contributions
I.P. performed all the experiments, and J.P. and G.K. collaborated on data analysis and project planning.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary table S1 (PDF 183 kb)
Rights and permissions
About this article
Cite this article
Peng, J., Karpen, G. H3K9 methylation and RNA interference regulate nucleolar organization and repeated DNA stability. Nat Cell Biol 9, 25–35 (2007). https://doi.org/10.1038/ncb1514
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1514
This article is cited by
-
Establishment of H3K9-methylated heterochromatin and its functions in tissue differentiation and maintenance
Nature Reviews Molecular Cell Biology (2022)
-
Two-step regulation of centromere distribution by condensin II and the nuclear envelope proteins
Nature Plants (2022)
-
The landscape of aging
Science China Life Sciences (2022)
-
Stabilization of heterochromatin by CLOCK promotes stem cell rejuvenation and cartilage regeneration
Cell Research (2021)
-
Did Cyclic Metaphosphates Have a Role in the Origin of Life?
Origins of Life and Evolution of Biospheres (2021)