Abstract
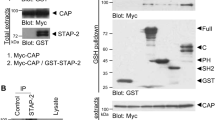
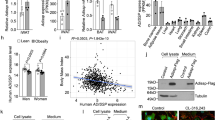
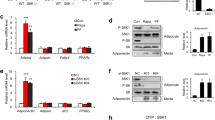
Adiponectin, also known as Acrp30, is an adipose tissue-derived hormone with anti-atherogenic, anti-diabetic and insulin sensitizing properties1,2,3. Two seven-transmembrane domain-containing proteins, AdipoR1 and AdipoR2, have recently been identified as adiponectin receptors4, yet signalling events downstream of these receptors remain poorly defined. By using the cytoplasmic domain of AdipoR1 as bait, we screened a yeast two-hybrid cDNA library derived from human fetal brain. This screening led to the identification of a phosphotyrosine binding domain and a pleckstrin homology domain-containing adaptor protein, APPL1 (adaptor protein containing pleckstrin homology domain, phosphotyrosine binding (PTB) domain and leucine zipper motif). APPL1 interacts with adiponectin receptors in mammalian cells and the interaction is stimulated by adiponectin. Overexpression of APPL1 increases, and suppression of APPL1 level reduces, adiponectin signalling and adiponectin-mediated downstream events (such as lipid oxidation, glucose uptake and the membrane translocation of glucose transport 4 (GLUT4)). Adiponectin stimulates the interaction between APPL1 and Rab5 (a small GTPase) interaction, leading to increased GLUT4 membrane translocation. APPL1 also acts as a critical regulator of the crosstalk between adiponectin signalling and insulin signalling pathways. These results demonstrate a key function for APPL1 in adiponectin signalling and provide a molecular mechanism for the insulin sensitizing function of adiponectin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Berg, A. H., Combs, T. P. & Scherer, P. E. ACRP30–adiponectin: an adipokine regulating glucose and lipid metabolism. Trends. Endocrinol. Metab. 13, 84–89 (2002).
Kadowaki, T. & Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 26, 439–451 (2005).
Tsao, T. S., Lodish, H. F. & Fruebis, J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur. J.Pharmacol. 440, 213–221 (2002).
Yamauchi, T. et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769 (2003).
Kubota, N. et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 277, 25863–25866 (2002).
Maeda, N. et al. Diet-induced insulin resistance in mice lacking adiponectin–ACRP30. Nature Med. 8, 731–737 (2002).
Yamauchi, T. et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Med. 7, 941–946 (2001).
Hu, E., Liang, P. & Spiegelman, B. M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol.Chem. 271, 10697–10703 (1996).
Weyer, C. et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 86, 1930–1935 (2001).
Statnick, M. A. et al. Decreased expression of apM1 in omental and subcutaneous adipose tissue of humans with type 2 diabetes. Int. J. Exp. Diabetes Res. 1, 81–88 (2000).
Hotta, K. et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 20, 1595–1599 (2000).
Liu, X. et al. A novel ability of Smad3 to regulate proteasomal degradation of a Cas family member HEF1. EMBO J. 19, 6759–6769 (2000).
Mitsuuchi, Y. et al. Identification of a chromosome 3p14.3–21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene 18, 4891–4898 (1999).
Liu, J. et al. Mediation of the DCC apoptotic signal by DIP13α. J. Biol. Chem. 277, 26281–26285 (2002).
Nechamen, C. A. et al. Human follicle-stimulating hormone (FSH) receptor interacts with the adaptor protein APPL1 in HEK 293 cells: potential involvement of the PI3K pathway in FSH signaling. Biol. Reprod. 71, 629–636 (2004).
Tomas, E. et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc. Natl Acad. Sci. USA 99, 16309–16313 (2002).
Yamauchi, T. et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Med. 8, 1288–1295 (2002).
Ceddia, R. B. et al. Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia 48, 132–139 (2005).
Miaczynska, M. et al. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 116, 445–456 (2004).
Huang, J., Imamura, T. & Olefsky, J. M. Insulin can regulate GLUT4 internalization by signaling to Rab5 and the motor protein dynein. Proc. Natl Acad. Sci. USA 98, 13084–13089 (2001).
Chen, X. & Wang, Z. Regulation of epidermal growth factor receptor endocytosis by wortmannin through activation of Rab5 rather than inhibition of phosphatidylinositol 3-kinase. EMBO Rep. 2, 842–849 (2001).
Dong, L. Q. et al. Phosphorylation of PKN by PDK1 mediates insulin signals to the actin cytoskeleton. Proc. Natl Acad. Sci. USA 97, 5089–5094 (2000).
Rother, K. I. et al. Evidence that IRS-2 phosphorylation is required for insulin action in hepatocytes. J. Biol. Chem. 273, 17491–17497 (1998).
Langlais, P. et al. Negative regulation of insulin-stimulated MAP kinase signaling by Grb10. Mol. Endocrinol. 18, 350–358 (2004).
Wong, G. W., Wang, J., Hug, C., Tsao, T. S. & Lodish, H. F. A family of Acrp30–adiponectin structural and functional paralogs. Proc. Natl Acad. Sci. USA 101, 10302–10307 (2004).
Somwar, R. et al. GLUT4 translocation precedes the stimulation of glucose uptake by insulin in muscle cells: potential activation of GLUT4 via p38 mitogen-activated protein kinase. Biochem. J. 359, 639–649 (2001).
Arai, M. et al. ConPred II: a consensus prediction method for obtaining transmembrane topology models with high reliability. Nucleic Acids Res. 32, W390–W393 (2004).
Acknowledgements
We thank M. A. Lim for generation of the APPL1 RNAi-suppressed cell lines, D. Hu and M, Chung for excellent technical assistance and V. Frohlich (Digital Optical Imaging Facility, UTHSCSA) for assistance with confocal microscopy studies. We also thank T. W. Wang for providing the yeast two-hybrid cDNA library, D. Accili for mouse hepatocyte cells, X. Y. Huang for wild-type and dominant negative (S34N) Rab5, J. H. Han for p38 MAPK constructs and M. J. Quon for the HA–GLUT4 and AMPK constructs. This work was supported in part by a Career Development Award from the American Diabetes Association (L.Q.D.) and National Institute of Health grants RO1 DK69930 (L.Q.D.), RO1 DK52933 (F.L.), pre-doctoral fellowship F31DK068874 (R.A.R.) and training grant T32 AG021890 (F.J.R. and J.Y.H.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3 and S4 (PDF 1208 kb)
Rights and permissions
About this article
Cite this article
Mao, X., Kikani, C., Riojas, R. et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8, 516–523 (2006). https://doi.org/10.1038/ncb1404
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1404
This article is cited by
-
The role of bidirectional communication between the adipokines and the endogenous opioid system in an experimental mouse model of colitis-associated colorectal cancer
Pharmacological Reports (2024)
-
MicroRNA-668-3p inhibits myoblast proliferation and differentiation by targeting Appl1
BMC Genomics (2023)
-
Impairment of APPL1/Myoferlin facilitates adipogenic differentiation of mesenchymal stem cells by blocking autophagy flux in osteoporosis
Cellular and Molecular Life Sciences (2022)
-
Transcriptomic analysis of patients with clinical suspicion of maturity-onset diabetes of the young (MODY) with a negative genetic diagnosis
Orphanet Journal of Rare Diseases (2022)
-
Paradigm shift: the primary function of the “Adiponectin Receptors” is to regulate cell membrane composition
Lipids in Health and Disease (2021)