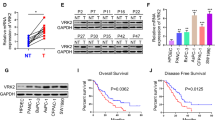
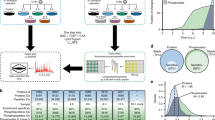
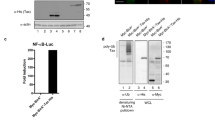
Abstract
Protein tyrosine phosphatases regulate important processes in eukaryotic cells and have critical functions in many human diseases including diabetes to cancer1,2,3. Here, we report that the human Vaccinia H1-related (VHR) dual-specific protein tyrosine phosphatase regulates cell-cycle progression and is itself modulated during the cell cycle. Using RNA interference (RNAi), we demonstrate that cells lacking VHR arrest at the G1–S and G2–M transitions of the cell cycle and show the initial signs of senescence, such as flattening, spreading, appearance of autophagosomes, β-galactosidase staining and decreased telomerase activity. In agreement with this notion, cells lacking VHR were found to upregulate p21Cip–Waf1, whereas they downregulated the expression of genes for cell-cycle regulators, DNA replication, transcription and mRNA processing. Loss of VHR also caused a several-fold increase in serum-induced activation of its substrates, the mitogen-activated protein (MAP) kinases Jnk and Erk. VHR-induced cell-cycle arrest was dependent on this hyperactivation of Jnk and Erk, and was reversed by Jnk and Erk inhibition or knock-down. We conclude that VHR is required for cell-cycle progression as it modulates MAP kinase activation in a cell-cycle phase-dependent manner.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Alonso, A. et al. The set of genes encoding protein tyrosine phosphatase family members in the human genome. Cell 117, 699–720 (2004).
Andersen, J. N. et al. A genomic perspective on protein tyrosine phosphatases: gene structure, pseudogenes, and genetic disease linkage. FASEB J. 18, 8–13 (2004).
Mustelin, T., Vang, T. and Bottini, N. Protein tyrosine phosphatases and the immune response. Nature Rev. Immunol. 5, 43–57 (2005).
Alonso, A., Rojas, A., Godzik, A. and Mustelin, T. The dual-specific protein tyrosine phosphatase family. Top. Curr. Genet. 5, 333–358 (2003).
Dorfman, K. et al. Disruption of the erp/mkp-1 gene does not affect mouse development: normal MAP kinase activity in ERP/MKP-1-deficient fibroblasts. Oncogene 13, 925–931 (1996).
Ishibashi, T., Bottaro, D. P., Chan, A., Kiki, T. and Aaronson, S. A. Expression cloning of a human dual-specificity phosphatase. Proc. Natl Acad. Sci. USA 89, 12170–12174 (1992).
Guan, K. L., Bryoles, S. S. and Dixon, J. E. A Tyr/Ser protein phosphatase encoded by Vaccinia virus. Nature 350 359–362 (1991).
Todd, J. L., Tanner, K. G. and Denu, J. M. Extracellular regulated kinases (ERK) 1 and ERK2 are authentic substrates for the dual-specificity protein-tyrosine phosphatase VHR. A novel role in down-regulating the ERK pathway. J. Biol. Chem. 274, 13271–13280 (1999).
Alonso, A., Saxena, M., Williams, S. and Mustelin, T. Inhibitory role for dual-specificity phosphatase VHR in T cell antigen receptor and CD28-induced Erk and Jnk activation. J. Biol. Chem. 276, 4766–4771 (2001).
Alonso, A. et al. Tyrosine phosphorylation of VHR phosphatase by ZAP-70. Nature Immunol. 4, 44–48 (2003).
Alonso, A. et al. The minimal essential core of a cysteine-based protein tyrosine phosphatase revealed by a novel 16-kDa VH1-like phosphatase, VHZ. J. Biol. Chem. 279, 35768–35774 (2004).
Dimri, G. P. et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Kang, M. K. et al. Senescence-associated genes in normal human oral keratinocytes. Exp. Cell Res. 287, 272–281 (2003).
Stein, G. H., Drullinger, L. F., Robetorye, R. S., Pereira-Smith, O. M. and Smith, J. R. Senescent cells fail to express cdc2, cycA, and cycB in response to mitogen stimulation. Proc. Natl Acad. Sci. USA 88, 11012–11016 (1991).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. and Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Pumiglia, K. M. and Decker, S. J. Cell cycle arrest mediated by the MEK/mitogen-activated protein kinase pathway. Proc. Natl Acad. Sci. USA 94, 448–452 (1997).
Wang, W. et al. Sequential activation of the MEK-extracellular signal-regulated kinase and MKK3/6-p38 mitogen-activated protein kinase pathways mediates oncogenic ras-induced premature senescence. Mol. Cell Biol. 22, 3389–3403 (2002).
Alblas, J., Slager-Davidov, R., Steenbergh, P. H., Sussenbach, J. S. and van den Burg, B. The role of MAP kinase in TPA-mediated cell cycle arrest of human breast cancer cells. Oncogene 16, 131–139 (1998).
Tang, D. et al. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J. Biol. Chem. 277, 12710–12717 (2002).
Park, J.-I., Strock, C. J., Ball, D. W. and Nelkin, B. D. The Ras/Raf/MEK/extracellular signal-regulated kinase pathway induces autocrine–paracrine growth inhibition via the leukemia inhibitory factor/JAK/STAT pathway. Mol. Cell Biol. 23, 543–554 (2003).
Lahlou, H. et al. sst2 somatostatin receptor inhibits cell proliferation through Ras-, Rap1-, and B-Raf-dependent ERK2 activation. J. Biol. Chem. 278, 39356–39371 (2003).
Chau, A. S.-S. and Shibuya, E. K. Inactivation of p42 mitogen-activated protein kinase is required for exit from M-phase after cyclin destruction. J. Biol. Chem. 274, 32085–32090 (1999).
Tchou, W.-W., Yie, T.-A., Tan, T.-H., Rom, W. N. and Tchou-Wong, K.-M. Role of c-Jun N-terminal kinase 1 (JNK1) in cell cycle checkpoint activated by the protease inhibitor N-acetyl-leucinyl-leucinyl-norleucinal. Oncogene 18, 6974–6980 (1999).
Grosch, S. et al. Activation of c-Jun-N-terminal-kinase is crucial for the induction of a cell cycle arrest in human colon carcinoma cells caused by flurbiprofen enantiomers. FASEB J. 17, 1316–1318 (2003).
Xue, Y., Ramaswamy, N. T., Hong, X. and Pelling, J. C. Association of JNK1 with p21waf1 and p53: modulation of JNK1 activity. Mol. Carcinogen. 36, 38–44 (2003).
Jiang, W. et al. PRC1: a human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol. Cell 2, 877–885 (1998).
Eisen, M. B. and Brown, P. O. DNA arrays for analysis of gene expression. Methods Enzymol. 303, 179–205 (1999).
Saxena, M., Williams, S., Taskén, K. and Mustelin, T. Crosstalk between cAMP-dependent kinase and MAP kinase through hematopoietic protein tyrosine phosphatase (HePTP). Nature Cell Biol. 1, 305–311 (1999).
Huynh, H. et al. Control of vesicle fusion by a tyrosine phosphatase. Nature Cell. Biol. 6, 831–839 (2004).
Acknowledgements
This work was supported by Rotary International, the Van Beirs Foundation, Centre Anticancéreux près L'Université de Liège, the Spanish Ministry of Education and Culture, the Plan Nacional de Salud y Farmacia (SAF 2003-05194) and the National Institutes of Health (AI35603).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3 and S4, Supplementary Table 1 and 2 (PDF 3691 kb)
Rights and permissions
About this article
Cite this article
Rahmouni, S., Cerignoli, F., Alonso, A. et al. Loss of the VHR dual-specific phosphatase causescell-cycle arrest and senescence. Nat Cell Biol 8, 524–531 (2006). https://doi.org/10.1038/ncb1398
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1398
This article is cited by
-
Rae1-mediated nuclear export of Rnc1 is an important determinant in controlling MAPK signaling
Current Genetics (2018)
-
Deficiency in VHR/DUSP3, a suppressor of focal adhesion kinase, reveals its role in regulating cell adhesion and migration
Oncogene (2017)
-
Withaferin A abolishes the stem cell factor-stimulated pigmentation of human epidermal equivalents by interrupting the auto-phosphorylation of c-KIT in human melanocytes
Archives of Dermatological Research (2015)
-
DUSP3/VHR is a pro-angiogenic atypical dual-specificity phosphatase
Molecular Cancer (2014)
-
The regulatory roles of phosphatases in cancer
Oncogene (2014)