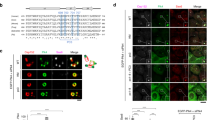
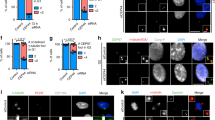
Abstract
Centrosome duplication is tightly controlled during faithful cell division, and unnecessary reduplication can lead to supernumerary centrosomes and multipolar spindles that are associated with most human cancer cells1,2,3,4,5. In addition to nucleocytoplasmic transport, the Ran–Crm1 network is involved in regulating centrosome duplication to ensure the formation of a bipolar spindle6,7,8. Here, we discover that nucleophosmin (NPM) may be a Ran–Crm1 substrate that controls centrosome duplication. NPM contains a functional nuclear export signal (NES) that is responsible for both its nucleocytoplasmic shuttling and its association with centrosomes, which are Ran–Crm1-dependent as they are sensitive to Crm1-specific nuclear export inhibition, either by leptomycin B (LMB) or by the expression of a Ran-binding protein, RanBP1. Notably, LMB treatment induces premature centrosome duplication in quiescent cells, which coincides with NPM dissociation from centrosomes. Moreover, deficiency of NPM by RNA interference results in supernumerary centrosomes, which can be reversed by reintroducing wild-type but not NES-mutated NPM. Mutation of a potential proline-dependent kinase phosphorylation site at residue 95, from threonine to aspartic acid (T95D) within the NES motif, abolishes NPM association and inhibition of centrosome duplication. Our results are consistent with the hypothesis that the Ran–Crm1 complex may promote a local enrichment of NPM on centrosomes, thereby preventing centrosome reduplication.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Sluder, G. & Hinchcliffe, E. H. Control of centrosome reproduction: the right number at the right time. Biol. Cell 91, 413–427 (1999).
Brinkley, B. R. Managing the centrosome numbers game: from chaos to stability in cancer cell division. Trends Cell Biol. 11, 18–21 (2001).
Nigg, E. A. Centrosome aberrations: cause or consequence of cancer progression? Nature Rev. Cancer 2, 815–825 (2002).
Doxsey, S. Duplicating dangerously: linking centrosome duplication and aneuploidy. Mol. Cell 10, 439–440 (2002).
Lingle, W. L. & Salisbury, J. L. The role of the centrosome in the development of malignant tumors. Curr. Top. Dev. Biol. 49, 313–329 (2000).
Forgues, M. et al. Involvement of Crm1 in hepatitis B virus X protein-induced aberrant centriole replication and abnormal mitotic spindles. Mol. Cell Biol. 23, 5282–5292 (2003).
Keryer, G. et al. Part of Ran is associated with AKAP450 at the centrosome: involvement in microtubule-organizing activity. Mol. Biol Cell 14, 4260–4271 (2003).
Di Fiore, B., Ciciarello, M. & Lavia, P. Mitotic functions of the Ran GTPase network: the importance of being in the right place at the right time. Cell Cycle 3, 305–313 (2004).
Dasso, M. Running on Ran: nuclear transport and the mitotic spindle. Cell 104, 321–324 (2001).
Weis, K. Regulating access to the genome: nucleocytoplasmic transport throughout the cell cycle. Cell 112, 441–451 (2003).
Di Fiore, B. et al. Mammalian RanBP1 regulates centrosome cohesion during mitosis. J. Cell Sci. 116, 3399–3411 (2003).
Kudo, N. et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl Acad. Sci. USA 96, 9112–9117 (1999).
Forgues, M. et al. Interaction of the hepatitis b virus x protein with the Crm1-dependent nuclear export pathway. J. Biol. Chem. 276, 22797–22803 (2001).
Tarapore, P., Okuda, M. & Fukasawa, K. A mammalian in vitro centriole duplication system: evidence for involvement of CDK2/cyclin E and nucleophosmin/B23 in centrosome duplication. Cell Cycle 1, 75–81 (2002).
Okuda, M. et al. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell 103, 127–140 (2000).
Borer, R. A., Lehner, C. F., Eppenberger, H. M. & Nigg, E. A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 56, 379–390 (1989).
Tokuyama, Y., Horn, H. F., Kawamura, K., Tarapore, P. & Fukasawa, K. Specific phosphorylation of nucleophosmin on Thr(199) by cyclin-dependent kinase 2-cyclin E and its role in centrosome duplication. J. Biol. Chem. 276, 21529–21537 (2001).
Zatsepina, O. V. et al. The nucleolar phosphoprotein B23 redistributes in part to the spindle poles during mitosis. J. Cell Sci. 112, 455–466 (1999).
Pinol-Roma, S. & Dreyfuss, G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature 355, 730–732 (1992).
Roth, J., Dobbelstein, M., Freedman, D. A., Shenk, T. & Levine, A. J. Nucleo-cytoplasmic shuttling of the hdm2 oncoprotein regulates the levels of the p53 protein via a pathway used by the human immunodeficiency virus rev protein. EMBO J. 17, 554–564 (1998).
Nishimura, Y., Ohkubo, T., Furuichi, Y. & Umekawa, H. Tryptophans 286 and 288 in the C-terminal region of protein B23.1 are important for its nucleolar localization. Biosci. Biotechnol. Biochem. 66, 2239–2242 (2002).
Moudjou, M. & Bornens, M. Cell Biology: A Laboratory Handbook (ed. Celis, J. E.) 111–119 (Academic Press, San Diego, 1998).
Itahana, K. et al. Tumor suppressor ARF degrades B23, a nucleolar protein involved in ribosome biogenesis and cell proliferation. Mol. Cell 12, 1151–1164 (2003).
Clarke, P. R. & Zhang, C. Ran GTPase: a master regulator of nuclear structure and function during the eukaryotic cell division cycle? Trends Cell Biol. 11, 366–371 (2001).
Stommel, J. M. et al. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 18, 1660–1672 (1999).
Colombo, E., Marine, J. C., Danovi, D., Falini, B. & Pelicci, P. G. Nucleophosmin regulates the stability and transcriptional activity of p53. Nature Cell Biol. 4, 529–533 (2002).
Butel, J. S. Viral carcinogenesis: revelation of molecular mechanisms and etiology of human disease. Carcinogenesis 21, 405–426 (2000).
Okuwaki, M., Tsujimoto, M. & Nagata, K. The RNA binding activity of a ribosome biogenesis factor, nucleophosmin/B23, is modulated by phosphorylation with a cell cycle-dependent kinase and by association with its subtype. Mol. Biol. Cell 13, 2016–2030 (2002).
Acknowledgements
We thank K. Nagata for the generous gift of GFP-tagged NPM, P. Lavia for the RanBP1 expression vector and J. Salisbury for anti-centrin antibody; C. Harris for invaluable comments; S. Garfield, S. Wincovitch and B. J. Taylor for superior technical support; K. MacPherson for bibliographical help; and the National Cancer Institute-Center for Cancer Research Fellows Editorial Board for editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1, S2, S3 and S4 (PDF 556 kb)
Rights and permissions
About this article
Cite this article
Wang, W., Budhu, A., Forgues, M. et al. Temporal and spatial control of nucleophosmin by the Ran–Crm1 complex in centrosome duplication. Nat Cell Biol 7, 823–830 (2005). https://doi.org/10.1038/ncb1282
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1282
This article is cited by
-
The nuclear lamina is a hub for the nuclear function of Jacob
Molecular Brain (2021)
-
HO-1 nuclear accumulation and interaction with NPM1 protect against stress-induced endothelial senescence independent of its enzymatic activity
Cell Death & Disease (2021)
-
Dipeptide repeat proteins inhibit homology-directed DNA double strand break repair in C9ORF72 ALS/FTD
Molecular Neurodegeneration (2020)
-
Functional impacts of the ubiquitin–proteasome system on DNA damage recognition in global genome nucleotide excision repair
Scientific Reports (2020)
-
Roniciclib down-regulates stemness and inhibits cell growth by inducing nucleolar stress in neuroblastoma
Scientific Reports (2020)