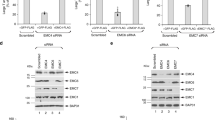
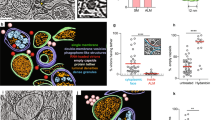
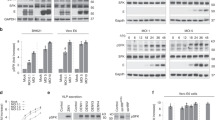
Abstract
During viral infection, fusion of the viral envelope with endosomal membranes and nucleocapsid release were thought to be concomitant events. We show here that for the vesicular stomatitis virus they occur sequentially, at two successive steps of the endocytic pathway. Fusion already occurs in transport intermediates between early and late endosomes, presumably releasing the nucleocapsid within the lumen of intra-endosomal vesicles, where it remains hidden. Transport to late endosomes is then required for the nucleocapsid to be delivered to the cytoplasm. This last step, which initiates infection, depends on the late endosomal lipid lysobisphosphatidic acid (LBPA) and its putative effector Alix/AIP1, and is regulated by phosphatidylinositol-3-phosphate (PtdIns(3)P) signalling via the PtdIns(3)P-binding protein Snx16. We conclude that the nucleocapsid is exported into the cytoplasm after the back-fusion of internal vesicles with the limiting membrane of late endosomes, and that this process is controlled by the phospholipids LBPA and PtdIns(3)P and their effectors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Gruenberg, J. The endocytic pathway: a mosaic of domains. Nature Rev. Mol. Cell Biol. 2, 721–730 (2001).
Katzmann, D.J., Odorizzi, G. & Emr, S.D. Receptor downregulation and multivesicular-body sorting. Nature Rev. Mol. Cell Biol. 3, 893–905 (2002).
Gruenberg, J. & Stenmark, H. The biogenesis of multivesicular endosomes. Nature Rev. Mol. Cell Biol. 5, 317–323 (2004).
Escola, J.M. et al. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 273, 20121–20127 (1998).
Griffiths, G., Hoflack, B., Simons, K., Mellman, I. & Kornfeld, S. The mannose-6-phosphate receptor and the biogenesis of lysosomes. Cell 52, 329–341 (1988).
Murk, J.L., Stoorvogel, W., Kleijmeer, M.J. & Geuze, H.J. The plasticity of multivesicular bodies and the regulation of antigen presentation. Semin. Cell Dev. Biol. 13, 303–311 (2002).
Kobayashi, T., Gu, F. & Gruenberg, J. Lipids, lipid domains and lipid-protein interactions in endocytic membrane traffic. Semin. Cell Dev. Biol. 9, 517–526 (1998).
Kobayashi, T. et al. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature 392, 193–197 (1998).
Galve-de Rochemonteix, B. et al. Interaction of anti-phospholipid antibodies with late endosomes of human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 20, 563–574 (2000).
Incardona, J.P., Gruenberg, J. & Roelink, H. Sonic hedgehog induces the segregation of Patched and Smoothened in endosomes. Curr. Biol. 12, 983–995 (2002).
Lebrand, C. et al. Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 21, 1289–1300 (2002).
Matsuo, H. et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303, 531–534 (2004).
Strack, B., Calistri, A., Craig, S., Popova, E. & Göttlinger, H.G. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114, 689–699 (2003).
von Schwedler, U.K. et al. The protein network of HIV budding. Cell 114, 701–713 (2003).
Whitney, J.A., Gomez, M., Sheff, D., Kreis, T.E. & Mellman, I. Cytoplasmic coat proteins involved in endosome function. Cell 83, 703–713 (1995).
Marsh, M., Bolzau, E. & Helenius, A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell 32, 931–940 (1983).
Pak, C.C., Puri, A. & Blumenthal, R. Conformational changes and fusion activity of vesicular stomatitis virus glycoprotein: [125I]iodonaphthyl azide photolabeling studies in biological membranes. Biochemistry 36, 8890–8896 (1997).
Lakadamyali, M., Rust, M.J., Babcock, H.P. & Zhuang, X. Visualizing infection of individual influenza viruses. Proc. Natl Acad. Sci. USA 100, 9280–9285 (2003).
Gruenberg, J., Griffiths, G. & Howell, K.E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J. Cell Biol. 108, 1301–1316 (1989).
Gruenberg, J. & Maxfield, F. Membrane transport in the endocytic pathway. Curr. Opin. Cell Biol. 7, 552–563 (1995).
Aniento, F., Emans, N., Griffiths, G. & Gruenberg, J. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J. Cell Biol. 123, 1373–1387 (1993).
Marsh, M. & Bron, R. SFV infection in CHO cells: cell-type specific restrictions to productive virus entry at the cell surface. J. Cell Sci. 110, 95–103 (1997).
Kobayashi, T. et al. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nature Cell Biol. 1, 113–118 (1999).
Naldini, L. et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267 (1996).
Fernandez-Borja, M. et al. Multivesicular body morphogenesis requires phosphatidyl-inositol 3-kinase activity. Curr. Biol. 9, 55–58 (1999).
Reaves, B.J., Bright, N.A., Mullock, B.M. & Luzio, J.P. The effect of wortmannin on the localisation of lysosomal type I integral membrane glycoproteins suggests a role for phosphoinositide 3-kinase activity in regulating membrane traffic late in the endocytic pathway. J. Cell Sci. 109, 749–762 (1996).
Futter, C.E., Collinson, L.M., Backer, J.M. & Hopkins, C.R. Human VPS34 is required for internal vesicle formation within multivesicular endosomes. J. Cell Biol. 155, 1251–1264 (2001).
Lloyd, T.E. et al. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108, 261–269 (2002).
Bache, K.G., Brech, A., Mehlum, A. & Stenmark, H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J. Cell Biol. 162, 435–442 (2003).
Khor, R., McElroy, L.J. & Whittaker, G.R. The ubiquitin-vacuolar protein sorting system is selectively required during entry of influenza virus into host cells. Traffic 4, 857–868 (2003).
Gillooly, D.J. et al. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 19, 4577–4588 (2000).
Petiot, A., Faure, J., Stenmark, H. & Gruenberg, J. PI3P signaling regulates receptor sorting but not transport in the endosomal pathway. J. Cell Biol. 162, 971–979 (2003).
Rhee, S.G. Regulation of phosphoinositide-specific phospholipase C. Annu. Rev. Biochem. 70, 281–312 (2001).
Sbrissa, D., Ikonomov, O.C. & Shisheva, A. Phosphatidylinositol 3-phosphate-interacting domains in PIKfyve. Binding specificity and role in PIKfyve. Endomembrane localization. J. Biol. Chem. 277, 6073–6079 (2002).
Carlton, J., Bujny, M., Rutherford, A. & Cullen, P. Sorting nexins — unifying trends and new perspectives. Traffic 6, 75–82 (2005).
Hanson, B.J. & Hong, W. Evidence for a role of SNX16 in regulating traffic between the early and later endosomal compartments. J. Biol. Chem. 278, 34617–34630 (2003).
Choi, J.H. et al. Sorting nexin 16 regulates EGF receptor trafficking by phosphatidylinositol-3-phosphate interaction with the Phox domain. J. Cell Sci. 117, 4209–4218 (2004).
Sinclair, A.M. et al. Interaction of vesicular stomatitis virus-G pseudotyped retrovirus with CD34+ and CD34+ CD38− hematopoietic progenitor cells. Gene Ther. 4, 918–927 (1997).
Chow, A., Toomre, D., Garrett, W. & Mellman, I. Dendritic cell maturation triggers retrograde MHC class II transport from lysosomes to the plasma membrane. Nature 418, 988–994 (2002).
Abrami, L., Lindsay, M., Parton, R.G., Leppla, S.H. & van der Goot, F.G. Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J. Cell Biol. 166, 645–651 (2004).
Odorizzi, G., Katzmann, D.J., Babst, M., Audhya, A. & Emr, S.D. Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae. J. Cell Sci. 116, 1893–1903 (2003).
Peter, B.J. et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, 495–499 (2004).
Habermann, B. The BAR-domain family of proteins: a case of bending and binding? EMBO Rep. 5, 250–255 (2004).
Kobayashi, T. et al. Separation and characterization of late endosomal membrane domains. J. Biol. Chem. 277, 32157–32164 (2002).
Elbashir, S.M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Clague, M.J., Urbe, S., Aniento, F. & Gruenberg, J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J. Biol. Chem. 269, 21–24 (1994).
Piguet, V. et al. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of β-COP in endosomes. Cell 97, 63–73 (1999).
Demaurex, N., Furuya, W., D'Souza, S., Bonifacino, J.S. & Grinstein, S. Mechanism of acidification of the trans-Golgi network (TGN). In situ measurements of pH using retrieval of TGN38 and furin from the cell surface. J. Biol. Chem. 273, 2044–2051 (1998).
Huber, L.A. et al. Both calmodulin and the unconventional myosin Myr4 regulate membrane trafficking along the recycling pathway of MDCK cells. Traffic 1, 494–503 (2000).
Parton, R.G., Schrotz, P., Bucci, C. & Gruenberg, J. Plasticity of early endosomes. J. Cell Sci. 103, 335–348 (1992).
Acknowledgements
We would like to thank B. Viaccoz, M.-C. Velluz and M.-H. Beuchat for expert technical assistance, and U. Laemmli and C. Bauer (Bioimaging platform, Frontiers in Genetics NCCR, Geneva) for help with light microscopy and imaging. We are grateful to K. Miwa and D. Trono for providing us with concanamycin B and HIV-1-derived vector pseudotyped with VSV-G, respectively. We also wish to thank G. van der Goot and M. Fivaz for critical reading of the manuscript. This work was supported by the Swiss National Science Foundation (J.G.), the International Human Frontier Science Program (J.G and R.G.P.), and the Australian National Health and Medical Research Council (R.G.P.). I.L.B. was supported by the award of the Bettencourt Foundation price (Prix des Jeunes Chercheurs), by the French Cancer Research Association (ARC) and by the Roche Foundation. V.P. was supported by the Roche Foundation and EMBO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figures S1 - S4, supplementary methods and movie legends (PDF 682 kb)
Rights and permissions
About this article
Cite this article
Le Blanc, I., Luyet, PP., Pons, V. et al. Endosome-to-cytosol transport of viral nucleocapsids. Nat Cell Biol 7, 653–664 (2005). https://doi.org/10.1038/ncb1269
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1269
This article is cited by
-
Lysosomal phospholipase A2 contributes to the biosynthesis of the atypical late endosome lipid bis(monoacylglycero)phosphate
Communications Biology (2023)
-
Antisense oligonucleotide activity in tumour cells is influenced by intracellular LBPA distribution and extracellular vesicle recycling
Communications Biology (2021)
-
Analysis of the dark proteome of Chandipura virus reveals maximum propensity for intrinsic disorder in phosphoprotein
Scientific Reports (2021)
-
Zebrafish C-reactive protein isoforms inhibit SVCV replication by blocking autophagy through interactions with cell membrane cholesterol
Scientific Reports (2020)
-
Where does the cargo go?: Solutions to provide experimental support for the “extracellular vesicle cargo transfer hypothesis”
Journal of Cell Communication and Signaling (2020)