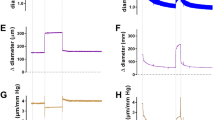
Abstract
The angiotensin II type 1 (AT1) receptor has a crucial role in load-induced cardiac hypertrophy. Here we show that the AT1 receptor can be activated by mechanical stress through an angiotensin-II-independent mechanism. Without the involvement of angiotensin II, mechanical stress not only activates extracellular-signal-regulated kinases and increases phosphoinositide production in vitro, but also induces cardiac hypertrophy in vivo. Mechanical stretch induces association of the AT1 receptor with Janus kinase 2, and translocation of G proteins into the cytosol. All of these events are inhibited by the AT1 receptor blocker candesartan. Thus, mechanical stress activates AT1 receptor independently of angiotensin II, and this activation can be inhibited by an inverse agonist of the AT1 receptor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Change history
25 June 2004
changed 'cardiomycytes' to 'cardiomyocytes'
References
Levy, D., Garrison, R.J., Savage, D.D., Kannel, W.B. & Castelli, W.P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N. Eng. J. Med. 322, 1561–1566 (1990).
Chien, K.R., Grace, A.A. & Hunter, J.J. Molecular biology of cardiac hypertrophy and heart failure. In Molecular Basis of Cardiovascular Disease (ed. K.R. Chien) 211–250 (W.B. Saunders, Philadelphia, PA, 1998).
Komuro, I. et al. Stretching cardiac myocytes stimulates protooncogene expression. J. Biol. Chem. 265, 3595–3598 (1990).
Sadoshima, J., Jahn, L., Takahashi, T., Kulik, T.J. & Izumo, S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J. Biol. Chem. 267, 10551–10560 (1992).
Komuro, I. & Yazaki, Y. Control of cardiac gene expression by mechanical stress. Annu. Rev. Physiol. 55, 55–75 (1993).
Sadoshima, J., Xu, Y., Slayter, H.S. & Izumo, S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 75, 977–984 (1993).
Yamazaki, T. et al. Angiotensin II partly mediates mechanical stress-induced cardiac hypertrophy. Circ. Res. 77, 258–265 (1995).
Kojima, M. et al. Angiotensin II receptor antagonist TCV-116 induces regression of hypertensive left ventricular hypertrophy in vivo and inhibits the intracellular signaling pathway of stretch-mediated cardiomyocyte hypertrophy in vitro. Circulation 89, 2204–2211 (1994).
Griendling, K.K., Lassegue, B. & Alexander, R.W. Angiotensin receptors and their therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 36, 281–306 (1996).
Pitt, B. et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial—the Losartan Heart Failure Survival Study ELITE II. Lancet 355, 1582–1587 (2000).
Cohn, J.N. et al. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N. Eng. J. Med. 345, 1667–1675 (2001).
Lindholm, L.H. et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359, 1004–1010 (2002).
Yamano, Y., Ohyama, K., Chaki, S., Guo, D.F. & Inagami, T. Identification of amino acid residues of rat angiotensin II receptor for ligand binding by site directed mutagenesis. Biochem. Biophys. Res. Comm. 187, 1426–1431 (1992).
Tanimoto, K. et al. Angiotensinogen-deficient mice with hypotension. J. Biol. Chem. 269, 31334–31337 (1994).
van Biesen, T., Luttrell, L.M., Hawes, B.E. & Lefkowitz, R.J. Mitogenic signaling via G protein-coupled receptors. Endocr. Rev. 17, 698–714 (1996).
Rockman, H.A., Koch, W.J. & Lefkowitz, R.J. Seven-transmembrane-spanning receptors and heart function. Nature 415, 206–212 (2002).
Bockaert, J. & Pin, J.P. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 18, 1723–1729 (1999).
Yamazaki, T. et al. Endothelin-1 is involved in mechanical stress-induced cardiomyocyte hypertrophy. J. Biol. Chem. 271, 3221–3228 (1996).
Zou, Y. et al. Both Gs and Gi proteins are critically involved in isoproterenol-induced cardiomyocyte hypertrophy. J. Biol. Chem. 274, 9760–9770 (1999).
Bernstein, K.E. & Alexander, R.W. Counterpoint: molecular analysis of the angiotensin II receptor. Endocr. Rev. 13, 381–386 (1992).
Inagami, T. Molecular biology and signaling of angiotensin receptors: an overview. J. Am. Soc. Nephrol. 11, S2–S7 (1999).
Seta, K., Nanamori, M., Modrall, J.G., Neubig, R.R. & Sadoshima, J. AT1 receptor mutant lacking heterotrimeric G protein coupling activates the Src–Ras–ERK pathway without nuclear translocation of ERKs. J. Biol. Chem. 277, 9268–9277 (2002).
Marrero, M.B. et al. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature 375, 247–250 (1995).
Ali, M.S. et al. Dependence on the motif YIPP for the physical association of Jak2 kinase with the intracellular carboxyl tail of the angiotensin II AT1 receptor. J. Biol. Chem. 272, 23382–23388 (1997).
Ali, M.S., Sayeski, P.P. & Bernstein, K.E. Jak2 acts as both a STAT1 kinase and as a molecular bridge linking STAT1 to the angiotensin II AT1 receptor. J. Biol. Chem. 275, 15586–15593 (2000).
Lee, M.A., Bohm, M., Paul, M. & Ganten, D. Tissue renin–angiotensin systems. Their role in cardiovascular disease. Circulation 87, 7–13 (1993).
Baker, K.M., Booz, G.W. & Dostal, D.E. Cardiac actions of angiotensin II: role of an intracardiac renin–angiotensin system. Annu. Rev. Physiol. 54, 227–241 (1992).
Mazzolai, L. et al. Increased cardiac angiotensin II levels induce right and left ventricular hypertrophy in normotensive mice. Hypertension 35, 985–991 (2000).
Wei, C.C. et al. Differential ANG II generation in plasma and tissue of mice with decrease expression of the ACE gene. Am. J. Physiol. 282, H2254–H2258 (2002).
Campbell, D.J. et al. Effect of reduced angiotensin-converting enzyme gene expression and angiotensin-converting enzyme inhibition on angiotensin and bradykinin peptide levels in mice. Hypertension 43, 1–6 (2004).
Knoll, R. et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell 111, 943–955 (2002).
Brancaccio, M. et al. Melusin, a muscle-specific integrin β1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nature Med. 9, 68–75 (2003).
Karnik, S.S., Gogonea, C., Patil, S., Saad, Y. & Takezako, T. Activation of G-protein-coupled receptors: a common molecular mechanism. Trends Endocrinol. Metab. 14, 431–437 (2004).
Akhter, S.A. et al. Targeting the receptor–Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science 280, 574–577 (1998).
Sano, T. et al. A domain for G protein coupling in carboxyl-terminal tail of rat angiotensin II receptor type 1A. J. Biol. Chem. 272, 23631–23636 (1997).
Lefkowitz, R.J., Cotecchia, S., Samama, P. & Costa, T. Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol. Sci. 14, 303–307 (1993).
Leurs, R., Smit, M.J., Alewijnse, A.E. & Timmerman, H. Agonist-independent regulation of constitutively active G-protein-coupled receptors. Trends Biochem. Sci. 23, 418–422 (1998).
Harada, K. et al. Acute pressure overload could induce hypertrophic responses in the heart of angiotensin II type 1a knockout mice. Circ. Res. 82, 779–785 (1998).
Kudoh, S. et al. Mechanical stretch induces hypertrophic responses in cardiac myocytes of angiotensin II type 1a receptor knockout mice. J. Biol. Chem. 273, 24037–24043 (1998).
Bader, M. et al. Tissue renin–angiotensin systems: new insights from experimental animal models in hypertension research. J. Mol. Med. 79, 76–102 (2001).
Ishida, J. et al. Expression and characterization of mouse angiotensin II type 1a receptor tagging hemagglutinin epitope in cultured cells. Int. J. Mol. Med. 3, 263–270 (1999).
Daaka, Y., Luttrell, L.M. & Lefkowitz, R.J. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 390, 88–91 (1997).
Sakurai, T. et al. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature 348, 732–735 (1990).
Sambrano, G.R. et al. Navigating the signalling network in mouse cardiac myocytes. Nature 420, 712–714 (2002).
Malhotra, R., Sadoshima, J., Brosius, F.C. & Izumo, S. Mechanical stretch and angiotensin II differentially upregulated the renin–angiotensin system in cardiac myocytes in vitro. Circ. Res. 85, 137–146 (1999).
Conklin, B.R., Chabre, O., Wong, Y.H., Federman, A.D. & Bourne, H.R. Recombinant Gqα. Mutational activation and coupling to receptors and phospholipase C. J. Biol. Chem. 267, 31–34 (1992)..
Iiri, T., Bell, S.M., Baranski, T.J., Fujita, T. & Bourne, H.R. A Gsα mutant designed to inhibit receptor signaling through Gs. Proc. Natl. Acad. Sci. USA 96, 499–504 (1999).
Garcia, P.D., Onrust, R., Bell, S.M., Sakmar, T.P. & Bourne, H.R. Transducin-α C-terminal mutations prevent activation by rhodopsin: a new assay using recombinant proteins expressed in cultured cells. EMBO J. 14, 4460–4469 (1995).
Acknowledgements
We thank R. J. Lefkowitz, J. Sadoshima and S. Kimura for plasmids; S.-i. Miura for advice; and A. Okubo, E. Fujita, R. Kobayashi and M. Watanabe for technical support. This work was supported by a Grant-in-Aid for Scientific Research, Developmental Scientific Research, and Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, and Culture; and by a grant for research on life science from Uehara Memorial Foundation, Japan (to I.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Zou, Y., Akazawa, H., Qin, Y. et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol 6, 499–506 (2004). https://doi.org/10.1038/ncb1137
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1137
This article is cited by
-
Mechanisms of endothelial flow sensing
Nature Cardiovascular Research (2023)
-
Promising Therapeutic Treatments for Cardiac Fibrosis: Herbal Plants and Their Extracts
Cardiology and Therapy (2023)
-
Interstitial-fluid shear stresses induced by vertically oscillating head motion lower blood pressure in hypertensive rats and humans
Nature Biomedical Engineering (2023)
-
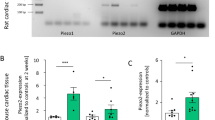
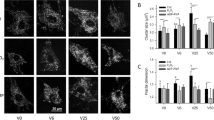
Piezo2 is not an indispensable mechanosensor in murine cardiomyocytes
Scientific Reports (2022)
-
Pleiotropic activation of endothelial function by angiotensin II receptor blockers is crucial to their protective anti-vascular remodeling effects
Scientific Reports (2022)