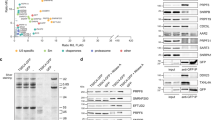
Abstract
The spliceosomal snRNPs U1, U2, U4 and U5 contain a common RNP structure termed the Sm core that is formed by the binding of Sm proteins onto the U snRNA. Although isolated Sm proteins assemble spontaneously onto U snRNAs in vitro, there is increasing evidence that SMN and its interactor Gemin2 are involved in this process in vivo. Here, we describe a cell-free assay system for the assembly of U snRNPs that closely reproduces in vivo conditions. Using this system, we show that assembly of U1 snRNP depends on ATP. Immunodepletion of SMN–Gemin2 from the extract abolished assembly even though the extract contained high levels of Sm proteins. An affinity-purified macromolecular SMN complex consisting of 16 components including all Sm proteins restored assembly in the immunodepleted extract. These data provide the first direct evidence that a complex containing SMN and Gemin2 mediates the active assembly of spliceosomal U snRNPs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Will, C. L. & Luhrmann, R. Spliceosomal UsnRNP biogenesis, structure and function. Curr. Opin. Cell Biol. 13, 290–301 (2001).
Achsel, T. et al. A doughnut-shaped heteromer of human Sm-like proteins binds to the 3′-end of U6 snRNA, thereby facilitating U4/U6 duplex formation in vitro. EMBO J. 18, 5789–5802 (1999).
Seraphin, B. Sm and Sm-like proteins belong to a large family: identification of proteins of the U6 as well as the U1, U2, U4 and U5 snRNPs. EMBO J. 14, 2089–2098 (1995).
Zhang, D., Abovich, N. & Rosbash, M. A biochemical function for the Sm complex. Mol. Cell 7, 319–329 (2001).
Mattaj, I. W. & De Robertis, E. M. Nuclear segregation of U2 snRNA requires binding of specific snRNP proteins. Cell 40, 111–118 (1985).
Mattaj, I. W. in Structure and Function of Major and Minor Small Nuclear Ribonculeoprotein Particles (ed. Birnstiel, M.) 100–114 (Springer-Verlag, Berlin/NewYork, 1988).
Neuman de Vegvar, H. E. & Dahlberg, J. E. Nucleocytoplasmic transport and processing of small nuclear RNA precursors. Mol. Cell. Biol. 10, 3365–3375 (1990).
Fischer, U. & Luhrmann, R. An essential signaling role for the m3G cap in the transport of U1 snRNP to the nucleus. Science 249, 786–790 (1990).
Raker, V. A., Plessel, G. & Luhrmann, R. The snRNP core assembly pathway: identification of stable core protein heteromeric complexes and an snRNP subcore particle in vitro. EMBO J. 15, 2256–2269 (1996).
Raker, V. A., Hartmuth, K., Kastner, B. & Lührmann, R. Spliceosomal U snRNP core assembly: Sm proteins assemble onto a Sm site RNA nonanucleotide in a specific and thermodynamically stable manner. Mol. Cell. Biol. 19, 6554–6565 (1999).
Fischer, U., Liu, Q. & Dreyfuss, G. The SMN–SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell 90, 1023–1029 (1997).
Buhler, D., Raker, V., Luhrmann, R. & Fischer, U. Essential role for the tudor domain of SMN in spliceosomal U snRNP assembly: implications for spinal muscular atrophy. Hum. Mol. Genet. 8, 2351–2357 (1999).
Liu, Q., Fischer, U., Wang, F. & Dreyfuss, G. The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 90, 1013–1021 (1997).
Charroux, B. et al. Gemin3: a novel DEAD box protein that interacts with SMN, the spinal muscular atrophy gene product, and is a component of gems. J. Cell Biol. 147, 1181–1194 (1999).
Grundhoff, A. T. et al. Characterisation of DP103, a novel DEAD box protein that binds to the Epstein–Barr virus nuclear proteins EBNA2 and EBNA3C. J. Biol. Chem. 27, 19136–19144 (1999).
Charroux, B. et al. Gemin4. A novel component of the SMN complex that is found in both gems and nucleoli. J. Cell Biol. 148, 1177–1186 (2000).
Meister, G. et al. Characterization of a nuclear 20S complex containing the survival of motor neurons (SMN) protein and a specific subset of spliceosomal Sm proteins. Hum. Mol. Genet. 9, 1977–1986 (2000).
Müller, B., Link, J. & Smythe, C. Assembly of U7 small nuclear ribonucleoprotein particle and histone RNA 3′ processing in Xenopus egg extracts. J. Biol. Chem. 275, 24284–24293 (2000).
Jarmolowski, A. & Mattaj, I. W. The determinants for Sm protein binding to Xenopus U1 and U5 snRNAs are complex and non-identical. EMBO J. 12, 223–232 (1993).
Hunt, S. L., Hsuan, J. J., Totty, N. & Jackson, R. J. unr, a cellular cytoplasmic RNA-binding protein with five cold-shock domains, is required for internal initiation of translation of human rhinovirus RNA. Genes Dev. 13, 347–448 (1998).
Johnson, J. L. & Craig, E. A. Protein folding in vivo: unraveling complex pathways. Cell 90, 201–204 (1997).
Pu, W. T., Krapivinsky, G. P., Krapivinsky, L. & Clapham, D. E. pICln inhibits snRNP biogenesis by binding core spliceosomal proteins. Mol. Cell. Biol. 19, 4113–4120 (1999).
Friesen, W. J., Massenet, S., Paushkin, S., Wyce, A. & Dreyfuss, G. SMN, the product of the spinal muscular atrophy gene, binds preferentially to dimethylarginine-containing protein targets. Mol. Cell 7, 1111–1117 (2001).
Friesen, W. J. & Dreyfuss, G. Specific sequences of the Sm and Sm-like (Lsm) proteins mediate their interaction with the spinal muscular atrophy disease gene product (SMN). J. Biol. Chem. 275, 26370–26375 (2000).
Liu, Q. & Dreyfuss, G. A novel nuclear structure containing the survival of motor neurons protein. EMBO J. 15, 3555–3565 (1996).
Jones, K. W. et al. Direct interaction of the spinal muscular atrophy disease protein SMN with the core snoRNP protein fibrillarin. J. Biol. Chem. (in the press).
Pellizzoni, L., Baccon, J., Charroux, B. & Dreyfuss, G. The survival of motor neurons (SMN) protein interacts with the snoRNP proteins fibrillarin and GAR1. Curr. Biol. 11, 1079–1088 (2001).
Murray, A. W. Cell cycle extracts. Methods Cell Biol. 36, 581–604 (1991).
Lerner, E. A., Lerner, M. R., Hardin, J. A., Janeway, C. A. & Steitz, J. A. Monoclonal antibodies to nucleic acid-containing cellular constituents: probes for molecular biology and autoimmune disease. Proc. Natl. Acad. Sci. USA 78, 2737–2741 (1981).
Acknowledgements
We are indebted to B. Laggerbauer and R. Terns for critically reading the manuscript, R. Lührmann and D. Schümperli for continuous discussion, O. Kelm and C. Kambach for providing reagents, and G. Sowa for technical support. This work was supported by the Boehringer Ingelheim Fonds (to D.B.), the Swiss National Science Foundation (no. 31-52619.97) (to R P.) and grants of the DFG (Fi-573/2-2).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary figures and table
Figure S1 The amino acid sequence of p175. (PDF 777 kb)
Figure S2 No de novo methylation of Sm proteins during assembly of U1 snRNP in vitro.
Figure S3 UV crosslink of SmG to U1 snRNA.
Table 1 Identification of three novel components of SMN complexes NSCII and CSC.
Rights and permissions
About this article
Cite this article
Meister, G., Bühler, D., Pillai, R. et al. A multiprotein complex mediates the ATP-dependent assembly of spliceosomal U snRNPs. Nat Cell Biol 3, 945–949 (2001). https://doi.org/10.1038/ncb1101-945
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1101-945
This article is cited by
-
The SMN complex drives structural changes in human snRNAs to enable snRNP assembly
Nature Communications (2023)
-
Sumoylation regulates the assembly and activity of the SMN complex
Nature Communications (2021)
-
SMN-deficiency disrupts SERCA2 expression and intracellular Ca2+ signaling in cardiomyocytes from SMA mice and patient-derived iPSCs
Skeletal Muscle (2020)
-
Nusinersen ameliorates motor function and prevents motoneuron Cajal body disassembly and abnormal poly(A) RNA distribution in a SMA mouse model
Scientific Reports (2020)
-
Functional characterization of SMN evolution in mouse models of SMA
Scientific Reports (2019)