Abstract
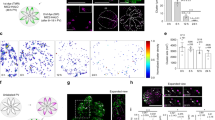
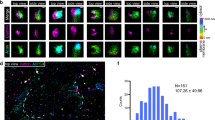
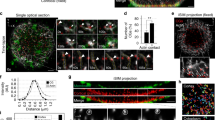
The actin filament (F-actin) cytoskeleton associates dynamically with the plasma membrane and is thus ideally positioned to participate in endocytosis. Indeed, a wealth of genetic and biochemical evidence has confirmed that actin interacts with components of the endocytic machinery1, although its precise function in endocytosis remains unclear. Here, we use 4D microscopy to visualize the contribution of actin during compensatory endocytosis in Xenopus laevis eggs. We show that the actin cytoskeleton maintains exocytosing cortical granules as discrete invaginated compartments, such that when actin is disrupted, they collapse into the plasma membrane. Invaginated, exocytosing cortical granule compartments are directly retrieved from the plasma membrane by F-actin coats that assemble on their surface. These dynamic F-actin coats seem to drive closure of the exocytic fusion pores and ultimately compress the cortical granule compartments. Active Cdc42 and N-WASP are recruited to exocytosing cortical granule membranes before F-actin coat assembly and coats assemble by Cdc42-dependent, de novo actin polymerization. Thus, F-actin may power fusion pore resealing and function in two novel endocytic capacities: the maintenance of invaginated compartments and the processing of endosomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Welch, M.D. & Mullins, R.D. Cellular control of actin nucleation. Annu. Rev. Cell Dev. Biol. 18, 247–288 (2002).
Geli, M.I. & Riezman, H. Endocytic internalization in yeast and animal cells: similar and different. J. Cell Sci. 111, 1031–1037 (1998).
Kaksonen, M., Peng, H.B. & Rauvala, H. Association of cortactin with dynamic actin in lamellipodia and on endosomal vesicles. J. Cell Sci. 113, 4421–4426 (2000).
Rozelle, A.L., et al. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr. Biol. 10, 311–320 (2000).
Schafer, D.A., D'Souza-Schorey, C. & Cooper, J.A. Actin assembly at membranes controlled by ARF6. Traffic. 1, 896–907 (2000).
Taunton, J. et al. Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J. Cell Biol. 148, 519–530 (2000).
Merrifield, C.J. et al. Annexin 2 has an essential role in actin-based macropinocytic rocketing. Curr. Biol. 11, 1136–1141 (2001).
Lee, E. & De Camilli, P. Dynamin at actin tails. Proc. Natl Acad. Sci. USA 99, 161–166 (2002).
Orth, J.D., Krueger, E.W., Cao, H. & McNiven, M.A. The large GTPase dynamin regulates actin comet formation and movement in living cells. Proc. Natl Acad. Sci. USA 99, 167–172 (2002).
Gundelfinger, E., Kessels, M. & Qualmann, B. Temporal and spatial coordination of exocytosis and endocytosis. Nature Rev. Mol. Cell Biol. 4, 127–139 (2003).
Merrifield, C.J., Feldman, M.E., Wan, L. & Almers, W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nature Cell Biol. 4, 691–698 (2002).
Merrifield, C.J. et al. Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nature Cell Biol. 1, 72–74 (1999).
Bement, W.M., Benink, H., Mandato, C.A. & Swelstad, B.B. Evidence for direct membrane retrieval following cortical granule exocytosis in Xenopus oocytes and eggs. J. Exp. Zool. 286, 767–775 (2000).
Whalley, T., Terasaki, M., Cho, M.S. & Vogel, S.S. Direct membrane retrieval into large vesicles after exocytosis in sea urchin eggs. J. Cell Biol. 131, 1183–1192 (1995).
Fesce, R., Grohovaz, F., Valtorta, F. & Meldolesi, J. Neurotransmitter release: fusion or 'kiss-and-run'? Trends Cell Biol. 4, 1–4 (1994).
Kline, D. & Nuccitelli, R. The wave of activation current in the Xenopus egg. Dev. Biol. 111, 471–487 (1985).
Terasaki, M., Runft, L.L. & Hand, A.R. Changes in organization of the endoplasmic reticulum during Xenopus oocyte maturation and activation. Mol. Biol. Cell 12, 1103–1116 (2001).
Cao, L.G. & Wang, Y.L. Mechanism of the formation of contractile ring in dividing cultured animal cells. I. Recruitment of preexisting actin filaments into the cleavage furrow. J. Cell Biol. 110, 1089–1095 (1990).
Mandato, C.A. & Bement, W.M. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J. Cell Biol. 154, 785–797 (2001).
De La Cruz, E. & Pollard, T. Transient kinetic analysis of rhodamine phalloidin binding to actin filaments. Biochemistry 33, 14387–14392 (1994).
Ma, L., Cantley, L.C., Janmey, P.A. & Kirschner, M.W. Corequirement of specific phosphoinositides and small GTP-binding protein Cdc42 in inducing actin assembly in Xenopus egg extracts. J. Cell Biol. 140, 1125–1136 (1998).
Moreau, V., & Way, M. Cdc42 is required for membrane-dependent actin polymerization in vitro. FEBS Lett. 427, 353–356 (1998).
Rohatgi, R., et al. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 97, 221–231 (1999).
Li, Z., Aizenman, C.D. & Cline, H.T. Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron 33, 741–750 (2002).
Higgs, H.N. & Pollard, T.D. Regulation of actin filament network formation through Arp2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 70, 649–676 (2001).
Kozma, R., Ahmed, S., Best, A. & Lim, L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell. Biol. 15, 1942–1952 (1995).
Bernheim-Groswasser, A., Wiesner, S., Golsteyn, R., Carlier, M. & Sykes, C. The dynamics of actin-based motility depend on surface parameters. Nature 417, 308–311 (2002).
Upadhyaya, A., Chabot, J.R., Andreeva, A., Samadani, A. & van Oudenaarden, A. Probing polymerization forces by using actin-propelled lipid vesicles. Proc. Natl Acad. Sci. USA 100, 4521–4526 (2003).
Giardini, P.A., Fletcher, D.A. & Theriot, J.A. Compression forces generated by actin comet tails on lipid vesicles. Proc. Natl Acad. Sci. USA 100, 6493–6498 (2003).
Becker, K.A. & Hart, N.H. Reorganization of filamentous actin and myosin-II in zebrafish eggs correlates temporally and spatially with cortical granule exocytosis. J. Cell Sci. 112, 97–110 (1999).
Lee, E. & Knecht, D.A. Visualization of actin dynamics during macropinocytosis and exocytosis. Traffic 3, 186–192 (2002).
Canman, J.C. & Bement, W.M. Microtubules suppress actomyosin-based cortical flow in Xenopus oocytes. J. Cell Sci. 110, 1907–1917 (1997).
Acknowledgements
We thank H. Higgs for providing the original WASP GBD clone and P. Krieg for the backbone Xenopus expression plasmid pCS2+. This work was supported by grants from the National Institutes of Health to both W.B. and J.T.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Sokac, A., Co, C., Taunton, J. et al. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat Cell Biol 5, 727–732 (2003). https://doi.org/10.1038/ncb1025
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1025
This article is cited by
-
Patterning of the cell cortex by Rho GTPases
Nature Reviews Molecular Cell Biology (2024)
-
Rho GTPase activity crosstalk mediated by Arhgef11 and Arhgef12 coordinates cell protrusion-retraction cycles
Nature Communications (2023)
-
Igf2bp3 maintains maternal RNA stability and ensures early embryo development in zebrafish
Communications Biology (2020)
-
Interaction of microtubules and actin during the post-fusion phase of exocytosis
Scientific Reports (2019)
-
Visualizing DC morphology and T cell motility to characterize DC-T cell encounters in mouse lymph nodes under mTOR inhibition
Science China Life Sciences (2019)