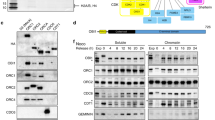
Abstract
To ensure proper timing of the G1–S transition in the cell cycle, the cyclin E–Cdk2 complex, which is responsible for the initiation of DNA replication, is restrained by the p21Cip1/p27Kip1/p57Kip2 family of CDK (cyclin-dependent kinase) inhibitors in humans and by the related p27Xic1 protein in Xenopus. Activation of cyclin E–Cdk2 is linked to the ubiquitination of human p27Kip1 or Xenopus p27Xic1 by SCF (for Skp1–Cullin–F-box protein) ubiquitin ligases. For human p27Kip1, ubiquitination requires direct phosphorylation by cyclin E–Cdk2. We show here that Xic1 ubiquitination does not require phosphorylation by cyclin E–Cdk2, but it does require nuclear accumulation of the Xic1–cyclin E–Cdk2 complex and recruitment of this complex to chromatin by the origin-recognition complex together with Cdc6 replication preinitiation factors; it also requires an activation step necessitating cyclin E–Cdk2-kinase and SCF ubiquitin-ligase activity, and additional factors associated with mini-chromosome maintenance proteins, including the inactivation of geminin. Components of the SCF ubiquitin-ligase complex, including Skp1 and Cul1, are also recruited to chromatin through cyclin E–Cdk2 and the preinitiation complex. Thus, activation of the cyclin E–Cdk2 kinase and ubiquitin-dependent destruction of its inhibitor are spatially constrained to the site of a properly assembled preinitiation complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Schwob, E., Böhm, T., Mendenhall, M. D. & Nasmyth, K. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. Cell 79, 233–244 (1994).
Deshaies, R. J. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Devel. Biol. 15, 435–467 (1999).
Carrano, A. C., Eytan, E., Hershko, A. & Pagano, M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature Cell Biol. 1, 193–199 (1999).
Montagnoli, A. et al. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 13, 1181–1189 (1999).
Nakayama, K. et al. Targeted disruption of skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 19, 2069–2081 (2000).
Sutterlüty, H. et al. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nature Cell Biol. 1, 207–214 (1999).
Yu, Z. K., Gervais, J. L. & Zhang, H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc. Natl Acad. Sci. USA 95, 11324–11329 (1998).
Sheaff, R. J. et al. Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination. Mol. Cell 5, 403–410 (2000).
Feldman, R. M., Correll, C. C., Kaplan, K. B. & Deshaies, R. J. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell 91, 221–230 (1997).
Verma, R. et al. Phosphorylation of Sic1p by G1 Cdk required for its degradation and entry into S phase. Science 278, 455–460 (1997).
Freed, E. et al. Components of an SCF ubiquitin ligase localize to the centrosome and regulate the centrosome duplication cycle. Genes Dev. 13, 2242–2257 (1999).
Yew, P. R. & Kirschner, M. W. Proteolysis and DNA replication: the CDC34 requirement in the Xenopus egg cell cycle. Science 277, 1672–1676 (1997).
Shou, W. & Dunphy, W. G. Cell cycle control by Xenopus p28Kix1, a developmentally regulated inhibitor of cyclin-dependent kinases. Mol. Biol. Cell 7, 457–469 (1996).
Su, J. Y., Rempel, R. E., Erikson, E. & Maller, J. L. Cloning and characterization of the Xenopus cyclin-dependent kinase inhibitor p27XIC1. Proc. Natl Acad. Sci. USA 92, 10187–10191 (1995).
Hartley, R. S., Sible, J. C., Lewellyn, A. L. & Maller, J. L. A role for cyclin E–Cdk2 in the timing of the midblastula transition in Xenopus embryos. Dev. Biol. 188, 312–321 (1997).
Ohnuma, S., Philpott, A., Wang, K., Holt, C. E. & Harris, W. A. p27Xic1, a Cdk inhibitor, promotes the determination of glial cells in Xenopus retina. Cell 99, 499–510 (1999).
Hardcastle, Z. & Papalopulu, N. Distinct effects of XBF-1 in regulating the cell cycle inhibitor p27(XIC1) and imparting a neural fate. Development 127, 1303–1314 (2000).
Swanson, C., Ross, J. & Jackson, P. K. Nuclear accumulation of cyclin E–Cdk2 triggers a concentration-dependent switch for the destruction of p27Xic1. Proc. Natl Acad. Sci. USA 97, 7796–7801 (2000).
Chuang, L.-C. & Yew, P. R. Regulation of the nuclear transport and degradation of the Xenopus cyclin-dependent kinase inhibitor, p27Xic1. J. Biol. Chem. 276, 1610–1617 (2001).
Furstenthal, L., Kaiser, B. K., Swanson, C. & Jackson, P. K. Cyclin E uses Cdc6 as a chromatin-associated receptor required for DNA replication. J. Cell Biol. 152, 1267–1278 (2001).
Bell, S. P. & Stillman, B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature 357, 128–134 (1992).
Rao, H. & Stillman, B. The origin recognition complex interacts with a bipartite DNA binding site within yeast replicators. Proc. Natl Acad. Sci. USA 92, 2224–2228 (1995).
Carpenter, P. B., Mueller, P. R. & Dunphy, W. G. Role for a Xenopus Orc2-related protein in controlling DNA replication. Nature 379, 357–360 (1996).
Coleman, T. R., Carpenter, P. B. & Dunphy, W. G. The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell 87, 53–63 (1996).
Maiorano, D., Moreau, J. & Méchali, M. XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature 404, 622–625 (2000).
Liang, C., Weinreich, M. & Stillman, B. ORC and Cdc6p interact and determine the frequency of initiation of DNA replication in the genome. Cell 81, 667–676 (1995).
Romanowski, P., Madine, M. A., Rowles, A., Blow, J. J. & Laskey, R. A. The Xenopus origin recognition complex is essential for DNA replication and MCM binding to chromatin. Curr. Biol. 6, 1416–1425 (1996).
Williams, R. S., Shohet, R. V. & Stillman, B. A human protein related to yeast Cdc6p. Proc. Natl Acad. Sci. USA 94, 142–147 (1997).
Yan, H., Gibson, S. & Tye, B. K. Mcm2 and Mcm3, two proteins important for ARS activity, are related in structure and function. Genes Dev. 5, 944–957 (1991).
McGarry, T. J. & Kirschner, M. W. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93, 1043–1053 (1998).
Tada, S., Li, A., Maiorano, D., Mechali, M. & Blow, J. J. Repression of origin assembly in metaphase depends on the inhibition of RFL-B/Cdt1 by geminin. Nature Cell Biol. 3, 107–113 (2001).
Wohlschlegel, J. A. et al. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science 290, 2309–2312 (2000).
Meijer, L. et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243, 527–536 (1997).
Hua, X. H., Yan, H. & Newport, J. A role for Cdk2 kinase in negatively regulating DNA replication during S phase of the cell cycle. J. Cell Biol. 137, 183–192 (1997).
Nishitani, H., Lygerou, Z., Nishimoto, T. & Nurse, P. The Cdt1 protein is required to license DNA for replication in fission yeast. Nature 404, 625–628 (2000).
Davydov, I. V., Patra, D. & Varshavsky, A. The N-end rule pathway in Xenopus egg extracts. Arch. Biochem. Biophys. 357, 317–325 (1998).
Wada, H., Yeh, E. T. & Kamitani, T. Identification of NEDD8-conjugation site in human cullin-2. Biochem. Biophys. Res. Commun. 257, 100–105 (1999).
Wu, K., Chen, A. & Pan, Z. Q. Conjugation of Nedd8 to CUL1 enhances the ability of the ROC1-CUL1 complex to promote ubiquitin polymerization. J. Biol. Chem. 275, 32317–32324 (2000).
Read, M. A. et al. Nedd8 modification of cul-1 activates SCF(β(TrCP))-dependent ubiquitination of IκBα. Mol. Cell. Biol. 20, 2326–2333 (2000).
Podust, V. N. et al. A Nedd8 conjugation pathway is essential for proteolytic targeting of p27Kip1 by ubiquitination. Proc. Natl Acad. Sci. USA 97, 4579–4584 (2000).
Morimoto, M., Nishida, T., Honda, R. & Yasuda, H. Modification of cullin-1 by ubiquitin-like protein Nedd8 enhances the activity of SCF(skp2) towards p27(kip1). Biochem. Biophys. Res. Commun. 270, 1093–1096 (2000).
Schulman, B. A., Lindstrom, D. L. & Harlow, E. Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc. Natl Acad. Sci. USA 95, 10453–10458 (1998).
Walter, J. C. Evidence for the sequential action of cdc7 and cdk2 protein kinases during initiation of DNA replication in Xenopus extracts. J. Biol. Chem. 275, 39773–39778 (2000).
Mimura, S. & Takisawa, H. Xenopus Cdc45-dependent loading of DNA polymerase-α onto chromatin under the control of S-phase Cdk. EMBO J. 17, 5699–5707 (1998).
Jares, P. & Blow, J. J. Xenopus cdc7 function is dependent on licensing but not on XORC, XCdc6, or CDK activity and is required for XCdc45 loading. Genes Dev. 14, 1528–1540 (2000).
Jaquenoud, M., Gulli, M. P., Peter, K. & Peter, M. The Cdc42p effector Gic2p is targeted for ubiquitin-dependent degradation by the SCFGrr1 complex. EMBO J. 17, 5360–5373 (1998).
Lacey, K. R., Jackson, P. K. & Stearns, T. Cyclin-dependent kinase control of centrosome duplication. Proc. Natl Acad. Sci. USA 96, 2817–2822 (1999).
Murray, A. W. & Kirschner, M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature 339, 275–280 (1989).
Murray, A. W. Cell cycle extracts. Methods Cell Biol. 36, 581–605 (1991).
Rexach, M. & Blobel, G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell 83, 683–692 (1995).
Jackson, P. K., Chevalier, S., Philippe, M. & Kirschner, M. W. Early events in DNA replication require cyclin E and are blocked by p21CIP1. J. Cell Biol. 130, 755–769 (1995).
Acknowledgements
We would like to thank A. Dutta, P. R. Yew, J. Maller, M. Rexach and R. Laskey for their generous gifts of reagents, and T. Coleman, P. Carpenter and D. Wolf for critical reading of the manuscript. This work was supported by grants from the National Institutes of Health (to P.K.J.), the National Cancer Institute (to L.F. and B.K.K.), Howard Hughes Medical Institute (to A.G.E.) and a Lieberman Fellowship (to B.K.K.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Furstenthal, L., Swanson, C., Kaiser, B. et al. Triggering ubiquitination of a CDK inhibitor at origins of DNA replication. Nat Cell Biol 3, 715–722 (2001). https://doi.org/10.1038/35087026
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35087026
This article is cited by
-
Selumetinib suppresses cell proliferation, migration and trigger apoptosis, G1 arrest in triple-negative breast cancer cells
BMC Cancer (2016)
-
MEK inhibitor effective against proliferation in breast cancer cell
Tumor Biology (2014)
-
SUMO2/3 modification of cyclin E contributes to the control of replication origin firing
Nature Communications (2013)
-
Molecular architecture of the DNA replication origin activation checkpoint
The EMBO Journal (2010)
-
P21Cip1/WAF1 downregulation is required for efficient PCNA ubiquitination after UV irradiation
Oncogene (2006)