Abstract
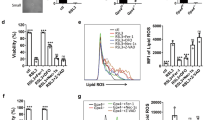
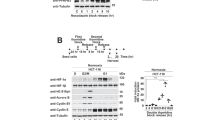
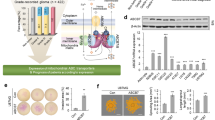
Haem oxygenase-1 (HO1) is a heat-shock protein that is induced by stressful stimuli. Here we demonstrate a cytoprotective role for HO1: cell death produced by serum deprivation, staurosporine or etoposide is markedly accentuated in cells from mice with a targeted deletion of the HO1 gene, and greatly reduced in cells that overexpress HO1. Iron efflux from cells is augmented by HO1 transfection and reduced in HO1-deficient fibroblasts. Iron accumulation in HO1-deficient cells explains their death: iron chelators protect HO1-deficient fibroblasts from cell death. Thus, cytoprotection by HO1 is attributable to its augmentation of iron efflux, reflecting a role for HO1 in modulating intracellular iron levels and regulating cell viability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lindquist, S. & Craig, E. A. The heat-shock proteins. Annu. Rev. Genet. 22, 631–677 (1988).
Lindquist, S. The heat-shock response. Annu. Rev. Biochem. 55, 1151–1191 (1986).
Hartl, F. U. Molecular chaperones in cellular protein folding. Nature 381, 571–579 (1996).
Bukau, B. & Horwich, A. L. The Hsp70 and Hsp60 chaperone machines. Cell 92, 351–366 (1998).
Wu, C. Heat shock transcription factors: structure and regulation. Annu. Rev. Cell Dev. Biol. 11, 441–469 (1995).
Camhi, S. L., Alam, J., Otterbein, L., Sylvester, S. L. & Choi, A. M. Induction of heme oxygenase-1 gene expression by lipopolysaccharide is mediated by AP-1 activation. Am. J. Resp. Cell Mol. Biol. 13, 387–398 (1995).
Choi, A. M. & Alam, J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Resp. Cell Mol. Biol. 15, 9–19 (1996).
Levinson, W., Oppermann, H. & Jackson, J. Transition series metals and sulfhydryl reagents induce the synthesis of four proteins in eukaryotic cells. Biochim. Biophys. Acta 606, 170–180 (1980).
Keyse, S. M. & Tyrrell, R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc. Natl Acad. Sci. USA 86, 99–103 (1989).
Maines, M. D. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 2, 2557–2568 (1988).
Maines, M. D. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 37, 517–554 (1997).
Yoshinaga, T., Sassa, S. & Kappas, A. The occurrence of molecular interactions among NADPH-cytochrome c reductase, heme oxygenase, and biliverdin reductase in heme degradation. J. Biol. Chem. 257, 7786–7793 (1982).
Farinelli, S. E., Greene, L. A. & Friedman, W. J. Neuroprotective actions of dipyridamole on cultured CNS neurons. J. Neurosci. 18, 5112–5123 (1998).
Kroemer, G., Dallaporta, B. & Resche-Rigon, M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 60, 619–642 (1998).
Green, D. R. & Reed, J. C. Mitochondria and apoptosis. Science 281, 1309–1312 (1998).
Ankarcrona, M. et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron 15, 961–973 (1995).
Trakshel, G. M., Kutty, R. K. & Maines, M. D. Cadmium-mediated inhibition of testicular heme oxygenase activity: the role of NADPH-cytochrome c (P-450) reductase. Arch. Biochem. Biophys. 251, 175–187 (1986).
Poss, K. D. & Tonegawa, S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl Acad. Sci. USA 94, 10919–10924 (1997).
Mukhopadhyay, C. K., Mazumder, B., Lindley, P. F. & Fox, P. L. Identification of the prooxidant site of human ceruloplasmin: a model for oxidative damage by copper bound to protein surfaces. Proc. Natl Acad. Sci. USA 94, 11546–11551 (1997).
Ponka, P., Beaumont, C. & Richardson, D. R. Function and regulation of transferrin and ferritin. Semin. Hematol. 35, 35–54 (1998).
Eisenstein, R. S., Garcia-Mayol, D., Pettingell, W. & Munro, H. N. Regulation of ferritin and heme oxygenase synthesis in rat fibroblasts by different forms of iron. Proc. Natl Acad. Sci. USA 88, 688–692 (1991).
Zakhary, R. et al. Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc. Natl Acad. Sci. USA 93, 795–798 (1996).
McCord, J. M. Iron, free radicals, and oxidative injury. Semin. Hematol. 35, 5–12 (1998).
Meneghini, R. Iron homeostasis, oxidative stress, and DNA damage. Free Rad. Biol. Med. 23, 783–792 (1997).
Gunshin, H. et al. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388, 482–488 (1997).
Umbreit, J. N., Conrad, M. E., Moore, E. G. & Latour, L. F. Iron absorption and cellular transport: the mobilferrin/paraferritin paradigm. Semin. Hematol. 35, 13–26 (1998).
Stearman, R., Yuan, D. S., Yamaguchi-Iwai, Y., Klausner, R. D. & Dancis, A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science 271, 1552–1557 (1996).
Conrad, M. E. Introduction: iron overloading disorders and iron regulation. Semin. Hematol. 35, 1–4 (1998).
Bothwell, T. H., Charlton, R.W. & Motulsky, A.G. in The Metabolic and Molecular Basis of Inherited Disease (eds Scriver, C. R., Beaudet, A.L., Sly, W.S. & Valle, D.) 2237–2269 (McGraw-Hill, New York, 1995).
Tenhunen, R., Marver, H. S. & Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl Acad. Sci. USA 61, 748–755 (1968).
Tenhunen, R., Marver, H. S. & Schmid, R. The enzymatic conversion of hemoglobin to bilirubin. Trans. Assoc. Am. Physicians 82, 363–371 (1969).
Barañano, D . et al. Identification and characterization of an iron transporting ATPase. Gastroenterol. 116, A1188 (1999).
Soares, M. P. et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nature Med. 4, 1073–1077 (1998).
Verma, A., Hirsch, D. J., Glatt, C. E., Ronnett, G. V. & Snyder, S. H. Carbon monoxide: a putative neural messenger. Science 259, 381–384 (1993).
Zakhary, R. et al. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc. Natl Acad. Sci. USA 94, 14848–14853 (1997).
Miller, S. M. et al. Heme oxygenase 2 is present in interstitial cell networks of the mouse small intestine. Gastroenterol. 114, 239–244 (1998).
Dennery, P. A. et al. Oxygen toxicity and iron accumulation in the lungs of mice lacking heme oxygenase-2. J. Clin. Invest. 101, 1001–1011 (1998).
McCoubrey, W. K. Jr, Huang, T. J. & Maines, M. D. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur. J. Biochem. 247, 725–732 (1997).
Bredt, D. S., Ferris, C. D. & Snyder, S. H. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J. Biol. Chem. 267, 10976–10981 (1992).
Hockenbery, D., Nunez, G., Milliman, C., Schreiber, R. D. & Korsmeyer, S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature 348, 334–336 (1990).
Acknowledgements
We thank R. Seaforth for providing [55Fe]haemin, D. C. Dodson for secretarial assistance, and S. Tonegawa for initial supplies of HO1−/− mice. This work was supported by USPHS grant MH-18501 and Research Scientist Award DA-00074 to S.H.S., and a National Research Service Award (DA-05900) to D.E.B. C.D.F. has a Howard Hughes Fellowship for Physicians and H.W. is a Pew Fellow.
Correspondence and requests for materials should be addressed to S.H.S. or C.D.F.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferris, C., Jaffrey, S., Sawa, A. et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol 1, 152–157 (1999). https://doi.org/10.1038/11072
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/11072
This article is cited by
-
Heme Oxygenase 1 in Vertebrates: Friend and Foe
Cell Biochemistry and Biophysics (2022)
-
The different facets of heme-oxygenase 1 in innate and adaptive immunity
Cell Biochemistry and Biophysics (2022)
-
Fluid shear stress regulates placental growth factor expression via heme oxygenase 1 and iron
Scientific Reports (2021)
-
Iron homeostasis regulates maturation of tomato (climacteric) and capsicum (non-climacteric) fruits
Journal of Plant Biochemistry and Biotechnology (2021)
-
Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers
Nature Communications (2020)