Abstract
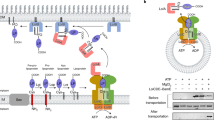
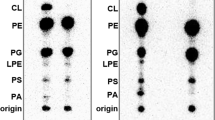
Lipoproteins in Escherichia coli are anchored to the periplasmic side of either the inner or the outer membrane by a lipid moiety that is covalently attached to the amino-terminal cysteine residue. Membrane specificity depends on a sorting signal at position 2 of the lipoprotein. Lipoproteins directed to the outer membrane are released from the inner membrane in an ATP-dependent manner through the formation of a complex with LolA, a periplasmic chaperone. However, the ATPase involved in this reaction has not been identified. Here we show, using reconstituted proteoliposomes, that a new complex, LolCDE, belonging to the ATP-binding cassette (ABC) transporter family, catalyses the release of lipoproteins in LolA- and sorting-signal-dependent manners. The LolCDE complex differs mechanistically from all other ABC transporters as it is not involved in the transmembrane transport of substrates. This new mechanism is evolutionarily conserved in other Gram-negative bacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hayashi, S. & Wu, H. C. Lipoproteins in bacteria. J. Bioenerg. Biomembr. 22, 451–471 (1990).
Pugsley, A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 57, 50–108 (1993).
Yamaguchi, K., Yu, F. & Inouye, M. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell 53, 423– 432 (1988).
Matsuyama, S., Tajima, T. & Tokuda, H. A novel periplasmic carrier protein involved in the sorting and transport of Escherichia coli lipoproteins destined for the outer membrane. EMBO J. 14, 3365– 3372 (1995).
Matsuyama, S., Yokota, N. & Tokuda, H. A novel outer membrane lipoprotein, LolB (HemM), involved in the LolA (p20)-dependent localization of lipoproteins to the outer membrane of Escherichia coli. EMBO J. 16, 6947–6955 (1997).
Tajima, T., Yokota, N., Matsuyama, S. & Tokuda, H. Genetic analyses of the in vivo function of LolA, a periplasmic chaperone involved in the outer membrane localization of Escherichia coli lipoproteins . FEBS Lett. 439, 51–54 (1998).
Yakushi, T., Yokota, N., Matsuyama, S. & Tokuda, H. LolA-dependent release of a lipid modified protein from the inner membrane of Escherichia coli requires nucleoside triphosphate. J. Biol. Chem. 273, 32576–32581 (1998).
Yakushi, T., Tajima, T., Matsuyama, S. & Tokuda, H. Lethality of the covalent linkage between mislocalized major outer membrane lipoprotein and the peptidoglycan of Escherichia coli. J. Bacteriol. 179, 2857–2862 (1997).
Yokota, N., Kuroda, T., Matsuyama, S. & Tokuda, H. Characterization of the LolA-LolB system as the general lipoprotein localization mechanism of Escherichia coli. J. Biol. Chem. 274, 30995–30999 (1999).
Walker, J. E., Saraste, M., Runswick, M. J. & Gay, N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1, 945–951 (1982).
Higgins, C. F. et al. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature 323, 448–450 (1986).
Linton, K. J. & Higgins, C. F. The Escherichia coli ATP-binding cassette (ABC) proteins. Mol. Microbiol. 28, 5–13 (1998).
Kohara, Y., Akiyama, K. & Isono, K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell 50, 495–508 (1987).
Yanisch-Perron, C., Vieira, J. & Messing, J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33, 103–119 (1985).
Hung, L.-W. et al. Crystal structure of the ATP-binding subunit of an ABC transporter . Nature 396, 703–707 (1998).
Shyamala, V., Baichwal, V., Beall, E. & Ames, G. F.-L. Structure-function analysis of the histidine permease and comparison with cystic fibrosis mutations . J. Biol. Chem. 266, 18714– 18719 (1991).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Tusnady, G. E. & Simon, I. Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J. Mol. Biol. 283, 489– 506 (1998).
Zhou, Z., White, K. A., Polissi, A., Georgopoulos, C. & Raetz, C. R. H. Function of Escherichia coli MsbA, an essential ABC family transporter, in lipid A and phospholipid biosynthesis. J. Biol. Chem. 273, 12466– 12475 (1998).
Higgins, C. F. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 ( 1992).
Tjalsma, H. et al. The role of lipoprotein processing by signal peptidase II in the Gram-positive eubacterium Bacillus subtilis. J. Biol. Chem. 274, 1698–1707 ( 1999).
Fraser, C. M. et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390, 580– 586 (1997).
Nishiyama, K., Fukuda, A., Morita, K. & Tokuda, H. Membrane-deinsertion of SecA underlying proton motive force-dependent stimulation of protein translocation . EMBO J. 18, 1049–1058 (1999).
Tokuda, H., Shiozuka, K. & Mizushima, S. Reconstitution of translocation activity for secretory proteins from solubilized components of Escherichia coli. Eur. J. Biochem. 192, 583–589 (1990).
Acknowledgements
We thank M. Wachi for E. coli DLP79-22 and R. Ishihara for secretarial support. The unfinished sequence data were provided by the Sanger center (Sty, Ype and Bpe), the University of Oklahoma (Aac and Ngo), the Institute of Genomic Research (Vch, Spu and Nme), the University of Washington Genome center and PathoGenesis Corporation (Pae), the University of Minnesota (Pmu) and the Washington University Genome Sequencing center (Kpn). This work was supported by CREST of the Japan Science and Technology Corporation, and grants from the Ministry of Education, Science, Sport and Culture of Japan to H.T.
Correspondence and requests for materials should be addressed to H.T.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yakushi, T., Masuda, K., Narita, Si. et al. A new ABC transporter mediating the detachment of lipid-modified proteins from membranes. Nat Cell Biol 2, 212–218 (2000). https://doi.org/10.1038/35008635
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35008635
This article is cited by
-
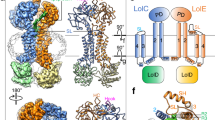
Structural basis for bacterial lipoprotein relocation by the transporter LolCDE
Nature Structural & Molecular Biology (2021)
-
Structure of a MacAB-like efflux pump from Streptococcus pneumoniae
Nature Communications (2018)
-
Subcellular localisation of lipoproteins of Vibrio vulnificus by the identification of outer membrane vesicles components
Antonie van Leeuwenhoek (2018)
-
Structural basis for lipopolysaccharide extraction by ABC transporter LptB2FG
Nature Structural & Molecular Biology (2017)
-
Development of a regulatable expression system for the functional study of Vibrio vulnificus essential genes
Antonie van Leeuwenhoek (2017)