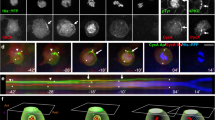
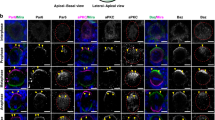
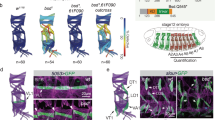
Abstract
The Drosophila protein Bazooka is required for both apical–basal polarity in epithelial cells and directing asymmetric cell division in neuroblasts. Here we show that the PDZ-domain protein DmPAR-6 cooperates with Bazooka for both of these functions. DmPAR-6 colocalizes with Bazooka at the apical cell cortex of epithelial cells and neuroblasts, and binds to Bazooka in vitro . DmPAR-6 localization requires Bazooka, and mislocalization of Bazooka through overexpression redirects DmPAR-6 to ectopic sites of the cell cortex. In the absence of DmPAR-6, Bazooka fails to localize apically in neuroblasts and epithelial cells, and is distributed in the cytoplasm instead. Epithelial cells lose their apical–basal polarity in DmPAR-6 mutants, asymmetric cell divisions in neuroblasts are misorientated, and the proteins Numb and Miranda do not segregate correctly into the basal daughter cell. Bazooka and DmPAR-6 are Drosophila homologues of proteins that direct asymmetric cell division in early Caenorhabditis elegans embryos, and our results indicate that homologous protein machineries may direct this process in worms and flies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Horvitz, H. R. & Herskowitz, I. Mechanisms of asymmetric cell division: two Bs or not two Bs, that is the question. Cell 68, 237–255 ( 1992).
Guo, S. & Kemphues, K. J. Molecular genetics of asymmetric cleavage in the early Caenorhabditis elegans embryo. Curr. Opin. Genet. Dev. 6, 408–415 (1996).
Knoblich, J. A. Mechanisms of asymmetric cell division during animal development. Curr. Opin. Cell Biol. 9, 833–841 (1997).
Lu, B., Jan, L. & Jan, Y. N. Control of cell divisions in the nervous system: symmetry and asymmetry. Annu. Rev. Neurosci. 23, 531–556 (2000).
Lin, H. & Schagat, T. Neuroblasts: a model for the asymmetric division of stem cells. Trends Genet. 13, 33–39 (1997).
Rhyu, M. S., Jan, L. Y. & Jan, Y. N. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells . Cell 76, 477–491 (1994).
Knoblich, J. A., Jan, L. Y. & Jan, Y. N. Asymmetric segregation of Numb and Prospero during cell division. Nature 377, 624– 627 (1995).
Shen, C. P., Jan, L. Y. & Jan, Y. N. Miranda is required for the asymmetric localization of Prospero during mitosis in Drosophila. Cell 90, 449–458 (1997).
Kraut, R., Chia, W., Jan, L. Y., Jan, Y. N. & Knoblich, J. A. Role of inscuteable in orienting asymmetric cell divisions in Drosophila. Nature 383, 50–55 (1996).
Kaltschmidt, J. A., Davidson, C. M., Brown, N. H. & Brand, A. H. Rotation and asymmetry of the mitotic spindle direct asymmetric cell division in the developing central nervous system. Nature Cell Biol. 2, 7–12 ( 2000).
Yu, F., Morin, X., Cai, Y., Yang, X. & Chia, W. Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in inscuteable apical localization. Cell 100, 399– 409 (2000).
Schaefer, M., Shevchenko, A. & Knoblich, J. A. A protein complex containing inscuteable and the gα-binding protein pins orients asymmetric cell divisions in drosophila. Curr. Biol. 10, 353– 362 (2000).
Parmentier, M. L. et al. Rapsynoid/Partner of Inscuteable controls asymmetric division of larval neuroblasts in Drosophila. J. Neurosci. (Online) 20, RC84 (2000).
Kuchinke, U., Grawe, F. & Knust, E. Control of spindle orientation in Drosophila by the Par-3-related PDZ-domain protein Bazooka. Curr. Biol. 8, 1357–1365 (1998).
Wodarz, A., Ramrath, A., Kuchinke, U. & Knust, E. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature 402, 544– 547 (1999).
Schober, M., Schaefer, M. & Knoblich, J. A. Bazooka recruits Inscuteable to orient asymmetric cell divisions in Drosophila neuroblasts. Nature 402 , 548–551 (1999).
Muller, H. A. & Wieschaus, E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 134, 149–163 (1996).
Kemphues, K. J., Priess, J. R., Morton, D. G. & Cheng, N. S. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 52, 311– 320 (1988).
Watts, J. L. et al. par-6, a gene involved in the establishment of asymmetry in early C. elegans embryos, mediates the asymmetric localization of PAR-3 . Development 122, 3133– 3140 (1996).
Tabuse, Y. et al. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development 125, 3607–3614 ( 1998).
Guo, S. & Kemphues, K. J. A non-muscle myosin required for embryonic polarity in Caenorhabditis elegans. Nature 382, 455–458 ( 1996).
Boyd, L., Guo, S., Levitan, D., Stinchcomb, D. T. & Kemphues, K. J. PAR-2 is asymmetrically distributed and promotes association of P granules and PAR-1 with the cortex in C. elegans embryos. Development 122, 3075 –3084 (1996).
Guo, S. & Kemphues, K. J. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 81, 611–620 (1995).
Hung, T. J. & Kemphues, K. J. PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development 126, 127 –135 (1999).
Etemad-Moghadam, B., Guo, S. & Kemphues, K. J. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 83, 743– 752 (1995).
Muller, H. A. Genetic control of epithelial cell polarity: lessons from Drosophila. Dev. Dyn. 218, 52–67 ( 2000).
Brand, A. H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
Lin, D. et al. A mammalian PAR-3–PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nature Cell Biol. 2, 540–547 (2000).
Qiu, R. G., Abo, A. & Steven Martin, G. A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Curr. Biol. 10, 697–707 (2000).
Joberty, G., Petersen, C., Gao, L. & Macara, I. G. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nature Cell Biol. 2, 531–539 (2000).
Wodarz, A., Ramrath, A., Grimm, A. & Knust, E. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J. Cell Biol. 150, 1361–1374 (2000).
Johansson, A., Driessens, M. & Aspenstrom, P. The mammalian homologue of the Caenorhabditis elegans polarity protein PAR-6 is a binding partner for the Rho GTPases; Cdc42 and Rac1. J. Cell Sci. 113, 3267– 3275 (2000).
Jantsch-Plunger, V. et al. CYK-4. A rho family gtpase activating protein (gap) required for central spindle formation and cytokinesis. J. Cell Biol. 149, 1391–1404 (2000).
Eaton, S., Auvinen, P., Luo, L., Jan, Y. N. & Simons, K. CDC42 and Rac1 control different actin-dependent processes in the Drosophila wing disc epithelium. J. Cell Biol. 131, 151–164 ( 1995).
Genova, J. L., Jong, S., Camp, J. T. & Fehon, R. G. Functional analysis of Cdc42 in actin filament assembly, epithelial morphogenesis, and cell signaling during Drosophila development. Dev. Biol. 221, 181–194 ( 2000).
Riggleman, B., Schedl, P. & Wieschaus, E. Spatial expression of the Drosophila segment polarity gene armadillo is posttranscriptionally regulated by wingless. Cell 63, 549– 560 (1990).
Dubreuil, R., Byers, T. J., Branton, D., Goldstein, L. S. & Kiehart, D. P. Drosophilia spectrin. I. Characterization of the purified protein. J. Cell Biol. 105, 2095–2102 ( 1987).
Tautz, D. & Pfeifle, C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback . Chromosoma 98, 81–85 (1989).
Chou, T. B. & Perrimon, N. Use of a yeast site-specific recombinase to produce female germline chimeras in Drosophila. Genetics 131, 643–653 ( 1992).
Acknowledgements
We want to thank M. Glotzer and A. Wodarz for discussion; K. Kemphues and T. Pawson for communicating results before publication; M. Glotzer for comments on the manuscript; and A. Wodarz, B. Chia, Y. N. Jan, D. St Johnston, the Developmental Studies Hybridoma Bank (DSHB) and the Bloomington Drosophila Stock Center for providing antibodies and flystocks.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petronczki, M., Knoblich, J. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat Cell Biol 3, 43–49 (2001). https://doi.org/10.1038/35050550
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35050550