Abstract
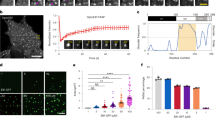
Microtubule assembly in Saccharomyces cerevisiae is initiated from sites within spindle pole bodies (SPBs) in the nuclear envelope. Microtubule plus ends are thought to be organized distal to the SPBs, while minus ends are proximal. Several hypotheses for the function of microtubule motor proteins in force generation and regulation of microtubule assembly propose that assembly and disassembly occur at minus ends as well as at plus ends. Here we analyse microtubule assembly relative to the SPBs in haploid yeast cells expressing green fluorescent protein fused to α-tubulin, a microtubule subunit. Throughout the cell cycle, analysis of fluorescent speckle marks on cytoplasmic astral microtubules reveals that there is no detectable assembly or disassembly at minus ends. After laser-photobleaching, metaphase spindles recover about 63% of the bleached fluorescence, with a half-life of about 1 minute. After anaphase onset, photobleached marks in the interpolar spindle are persistent and do not move relative to the SPBs. In late anaphase, the elongated spindles disassemble at the microtubule plus ends. These results show for astral and anaphase interpolar spindle microtubules, and possibly for metaphase spindle microtubules, that microtubule assembly and disassembly occur at plus, and not minus, ends.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hoyt, M. A. & Geiser, J. R. Genetic analysis of the mitotic spindle. Annu. Rev. Genet. 30, 7– 33 (1996).
Saunders, W. S. Action at the ends of microtubules. Curr. Opin. Cell Biol. 11, 129–133 (1999).
Carminati, J. L. & Stearns, T. Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J. Cell Biol. 138, 629– 641 (1997).
Shaw, S. L., Yeh, E., Maddox, P., Salmon, E. D. & Bloom, K. Astral microtubule dynamics in yeast: a microtubule-based searching mechanism for spindle orientation and nuclear migration into the bud. J. Cell Biol. 139, 985– 994 (1997).
Winey, M. et al. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J. Cell Biol. 129, 1601–1615 (1995).
Cottingham, F. R. & Hoyt, M. A. Mitotic spindle positioning in Saccharomyces cerevisiae is accomplished by antagonistically acting microtuble motor proteins. J. Cell Biol. 138 , 1041–1053 (1997).
Theesfeld, C. L., Irazoqui, J. E., Bloom, K. & Lew, D. J. The role of actin in spindle orientation changes during the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 146, 1019–1032 (1999).
Straight, A. F., Marshall, W. F. & Murray, A. W. Mitosis in living budding yeast: anaphase A but no metaphase plate. Science 277, 574– 578 (1997).
Straight, A. F., Sedat, J. W. & Murray, A. W. Time-lapse microscopy reveals unique roles for kinesins during anaphase in budding yeast. J. Cell Biol. 143 , 687–694 (1998).
Lee, L., Klee, S. K., Evangelista, M., Boone, C. & Pellman, D. Control of mitotic spindle position by the Saccharomyces cerevisiae formin Bni1p. J. Cell Biol. 144, 947–961 ( 1999).
Huyett, A., Kahana, J., Silver, P., Zeng, X. & Saunders, W. S. The Kar3p and Kip2p motors function antagonistically at the spindle poles to influence cytoplasmic microtubule numbers. J. Cell Sci. 111, 295–301 (1998).
Endow, S. A. et al. Yeast Kar3 is a minus-end microtubule motor protein that destabilizes microtubules preferentially at the minus ends. EMBO J. 13, 2708–2713 (1994).
Meluh, P. B. & Rose, M. D. KAR3, a kinesin-related gene required for yeast nuclear fusion. Cell 60, 1029– 1041 (1990).
DeZwaan, T. M., Ellingson, E., Pellman, D. & Roof, D. M. Kinesin-related KIP3 of Saccharomyces cerevisiae is required for a distinct step in nuclear migration. J. Cell Biol. 138, 1023–1040 (1997).
Desai, A. & Mitchison, T. J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 13, 83–117 (1997).
Waterman-Storer, C. M. & Salmon, E. D. How microtubules get fluorescent speckles. Biophys. J. 75, 2059–2069 (1998).
Salmon, E. D. et al. A high-resolution multimode digital microscope system. Methods Cell Biol. 56, 185–215 (1998).
Yeh, E., Skibbens, R. V., Cheng, J. W., Salmon, E. D. & Bloom, K. Spindle dynamics and cell cycle regulation of dynein in the budding yeast, Saccharomyces cerevisiae. J. Cell Biol. 130, 687–700 ( 1995).
Jacobs, C. W., Adams, A. E., Szaniszlo, P. J. & Pringle, J. R. Functions of microtubules in the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 107, 1409–1426 (1988).
Maddox, P. et al. Microtubule dynamics from mating through the first zygotic division in the budding yeast Saccharomyces cerevisiae. J. Cell Biol. 144, 977–987 ( 1999).
O’Toole, E. T., Winey, M. & MacIntosh, J. R. High-voltage electron tomography of spindle pole bodies and early mitotic spindles in the yeast Saccharomyces cerevisiae . Mol. Biol. Cell 10, 2017– 2031 (1999).
Wein, H., Foss, M., Brady, B. & Cande, W. Z. DSK1, a novel kinesin-related protein from the diatom Cylindrotheca fusiformis that is involved in anaphase spindle elongation. J. Cell Biol. 133, 595–604 (1996).
Saxton, W. M. & McIntosh, J. R. Interzone microtubule behavior in late anaphase and telophase spindles. J. Cell Biol. 105, 875–886 (1987).
Mallavarapu, A., Sawin, K. & Mitchison, T. A switch in microtubule dynamics at the onset of anaphase B in the mitotic spindle of Schizosaccharomyces pombe. Curr. Biol. 9, 1423–1426 (1999).
Sawin, K. E. & Mitchison, T. J. Microtubule flux in mitosis is independent of chromosomes, centrosomes, and antiparallel microtubules . Mol. Biol. Cell 5, 217– 226 (1994).
Mitchison, T. J. Polewards microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J. Cell Biol. 109, 637– 652 (1989).
Desai, A., Maddox, P. S., Mitchison, T. J. & Salmon, E. D. Anaphase A chromosome movement and poleward spindle microtubule flux occur at similar rates in Xenopus extract spindles. J. Cell Biol. 141, 703–713 (1998).
Waterman-Storer, C. M., Desai, A., Bulinski, J. C. & Salmon, E. D. Fluorescent speckle microscopy, a method to visualize the dynamics of protein assemblies in living cells. Curr. Biol. 8, 1227–1230 (1998).
Waterman-Storer, C. M. & Salmon, E. D. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J. Cell Biol. 139, 417–434 (1997).
Keating, T.J., Peloquin, J. G., Rodionov, V. I., Momcilovic, D. & Borisy, G. G. Microtubule release from the centrosome . Proc. Natl Acad. Sci USA 94, 5078– 5083 (1997).
Salmon, E. D. & Wadsworth, P. in Applications of Fluorescence in the Biomedical Sciences (eds Taylor, D. L., Waggoner, A. S., Lanni, F., Murphy, R. F. & Birge, R. R.) 377–403 (Alan R. Liss, New York, 1986).
Shaw, S. L., Yeh, E., Bloom, K. & Salmon, E. D. Imaging green fluorescent protein fusion proteins in Saccharomyces cerevisiae. Curr. Biol. 7, 701–704 ( 1997).
Acknowledgements
P.S.M. thanks members of the Salmon and Bloom laboratories for support and A. Straight for the gift of GFP–Tub1. We also thank Nikon, Hamamatsu Photonics and Universal Imaging for the loan of equipment used for development of our imaging systems. This work was supported by NIH grants GM32238 (to K.S.B.) and GM24364 (to E.D.S.).
Correspondence and requests for materials should be addressed to P.S.M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maddox, P., Bloom, K. & Salmon, E. The polarity and dynamics of microtubule assembly in the budding yeast Saccharomyces cerevisiae. Nat Cell Biol 2, 36–41 (2000). https://doi.org/10.1038/71357
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/71357
This article is cited by
-
Spatiotemporal control of spindle disassembly in fission yeast
Cellular and Molecular Life Sciences (2019)
-
Bidirectional motility of kinesin-5 motor proteins: structural determinants, cumulative functions and physiological roles
Cellular and Molecular Life Sciences (2018)
-
Regulation of Chromosome Speeds in Mitosis
Cellular and Molecular Bioengineering (2013)
-
Dynamics and functions of the actin cytoskeleton during the plant cell cycle
Chinese Science Bulletin (2011)
-
50 ways to build a spindle: the complexity of microtubule generation during mitosis
Chromosome Research (2011)