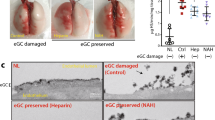
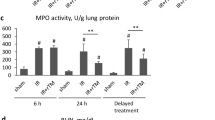
Abstract
Vascular immunotargeting may facilitate the rapid and specific delivery of therapeutic agents to endothelial cells. We investigated whether targeting of an antioxidant enzyme, catalase, to the pulmonary endothelium alleviates oxidative stress in an in vivo model of lung transplantation. Intravenously injected enzymes, conjugated with an antibody to platelet-endothelial cell adhesion molecule-1, accumulate in the pulmonary vasculature and retain their activity during prolonged cold storage and transplantation. Immunotargeting of catalase to donor rats augments the antioxidant capacity of the pulmonary endothelium, reduces oxidative stress, ameliorates ischemia-reperfusion injury, prolongs the acceptable cold ischemia period of lung grafts, and improves the function of transplanted lung grafts. These findings validate the therapeutic potential of vascular immunotargeting as a drug delivery strategy to reduce endothelial injury. Potential applications of this strategy include improving the outcome of clinical lung transplantation and treating a wide variety of endothelial disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gibbons, G. & Dzau, V. Molecular therapies for vascular diseases. Science 272, 689–693 (1996).
Langer, R. Drug delivery and targeting. Nature 392, 5–10 (1998).
Duo, L. & Saltzman, W.M. Synthetic DNA delivery systems. Nat. Biotechnol. 18, 33–37 (2000).
Reynolds, P. et al. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat. Biotechnol. 19, 898–842 (2001).
Muzykantov, V. Immunotargeting of drugs to the pulmonary vascular endothelium as a therapeutic strategy. Pathophysiology 5, 15–33 (1998).
Muzykantov, V.R. et al. Streptavidin facilitates internalization and pulmonary targeting of an anti-endothelial cell antibody (platelet-endothelial cell adhesion molecule 1): a strategy for vascular immunotargeting of drugs. Proc. Natl. Acad. Sci. USA 96, 2379–2384 (1999).
Li, S., Tan, Y., Viroonchatapan, E., Pitt, B.R. & Huang, L. Targeted gene delivery to pulmonary endothelium by anti-PECAM antibody. Am. J. Physiol. Lung Cell. Mol. Physiol. 278, L504–L511 (2000).
Scherpereel, A. et al. Cell-selective intracellular delivery of a foreign enzyme to endothelium in vivo using vascular immunotargeting. FASEB. J. 15, 416–426 (2001).
Scherpereel, A. et al. Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature. J. Pharmacol. Exp. Ther. 300, 777–786 (2002).
Eckenhoff, R.G., Dodia, C., Tan, Z. & Fisher, A.B. Oxygen-dependent reperfusion injury in the isolated rat lung. J. Appl. Physiol. 72, 1454–1460 (1992).
Eppinger, M.J., Deeb, G.M., Bolling, S.F. & Ward, P.A. Mediators of ischemia-reperfusion injury of rat lung. Am. J. Pathol. 150, 1773–1784 (1997).
Li, C. & Jackson, R.M. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am. J. Physiol. Cell. Physiol. 282, C227–C241 (2002).
Chabot, F., Mitchell, J.A., Gutteridge, J.M.C. & Evans, T.W. Reactive oxygen species in acute lung injury. Eur. Respir. J. 11, 745–757 (1998).
Novick, R.J., Gehman, K.E., Ali, I.S. & Lee, J. Lung preservation: the importance of endothelial and alveolar type II cell integrity. Ann. Thorac. Surg. 62, 302–314 (1996).
King, R.C. et al. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann. Thorac. Surg. 69, 1681–1685 (2000).
Meyers, B.F. et al. Lung transplantation: a decade of experience. Ann. Surg. 230, 362–371 (1999).
Christie, J.D. et al. Primary graft failure following lung transplantation. Chest. 114, 51–60 (1998).
Kelly, R.F. Current strategies in lung preservation. J. Lab. Clin. Med. 136, 427–440 (2000).
Muzykantov, V.R. Targeting of superoxide dismutase and catalase to vascular endothelium. J. Control. Release 71, 1–21 (2001).
Christofidou-Solomidou, M. et al. Vascular immunotargeting of glucose oxidase to the endothelial antigens induces distinct forms of oxidant acute lung injury. Am. J. Pathol. 160, 1155–1169 (2002).
Muzykantov, V.R., Atochina, E.N., Ischiropoulos, H., Danilov, S.M. & Fisher, A.B. Immunotargeting of antioxidant enzymes to the pulmonary endothelium. Proc. Natl. Acad. Sci. USA 93, 5213–5218 (1996).
Danilov, S.M. et al. Lung uptake of antibodies to endothelial antigens: key determinants of vascular immunotargeting. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L1335–L1347 (2001).
Spragg, D.D. et al. Immunotargeting of liposomes to activated vascular endothelial cells: a strategy for site-selective delivery in the cardiovascular system. Proc. Natl. Acad. Sci. USA 94, 8795–8800 (1997).
Maruyama, K., Kennel, S.J. & Huang, L. Lipid composition is important for highly efficient target binding and retention of immunoliposomes. Proc. Natl. Acad. Sci. USA 87, 5744–5748 (1990).
Rajotte, D. et al. Molecular heterogeneity of the vascular endothelium revealed by in vivo phage display. J. Clin. Invest. 102, 430–437 (1998).
McIntosh, D.P., Tan, X.Y., Oh, P. & Schnitzer, J.E. Targeting endothelium and its dynamic caveolae for tissue-specific transcytosis in vivo: a pathway to overcome cell barriers to drug and gene delivery. Proc. Natl. Acad. Sci. USA 99, 1996–2001 (2002).
Davis, M.G. & Hagen, P-O. The vascular endothelium. Ann. Surg. 218, 593–609 (1993).
Newman, P.J. The biology of PECAM-1. J. Clin. Invest. 99, 3–8 (1997).
Wiewrodt, R. et al. Size-dependant intracellular immunotargeting of therapeutic cargoes into endothelial cells. Blood 99, 912–922 (2002).
Christofidou-Solomidou, M. et al. Immunotargeting of glucose oxidase to endothelium in vivo causes oxidative vascular injury in the lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 278, L794–L805 (2000).
Williams, A. et al. Compromised antioxidant status and persistent oxidative stress in lung transplant recipients. Free Radic. Res. 30, 383–393 (1999).
Freeman, B.A. & Crapo, J.D. Biology of disease: free radicals and tissue injury. Lab. Invest. 47, 412–426 (1982).
Al-Mehdi, A.B. et al. Endothelial NADPH oxidase as the source of oxidants in lungs exposed to ischemia or high K+. Circ. Res. 83, 730–737 (1998).
Siflingerbirnboim, A. & Malik, A.B. Neutrophil adhesion to endothelial cells impairs the effects of catalase and glutathione in preventing endothelial injury. J. Cell. Physiol. 155, 234–239 (1993).
Turrens, J.F., Crapo, J.D. & Freeman, B.A. Protection against oxygen toxicity by intravenous injection of liposome-entrapped catalase and superoxide dismutase. J. Clin. Invest. 73, 87–95 (1984).
Yabe, Y., Nishikawa, M., Tamada, A., Takakura, Y. & Hashida, M. Targeted delivery and improved therapeutic potential of catalase by chemical modification: combination with superoxide dismutase derivatives. J. Pharmacol. Exp. Ther. 289, 1176–1184 (1999).
Corvo, M. et al. Intravenous administration of superoxide dismutase entrapped in long circulating liposomes. II. In vivo fate in a rat model of adjuvant arthritis. Biochem. Biophys. Acta. 77633, 1–10 (1999).
Vaporciyan, A.A. et al. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science 262, 1580–1582 (1993).
Bogen, S., Pak, J., Garifallou, M., Deng, X. & Muller, W.A. Monoclonal antibody to murine PECAM-1 (CD31) blocks acute inflammation in vivo. J. Exp. Med. 179, 1059–1064 (1994).
Fujita, T. et al. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by de-repression of fibrinolysis. Nat. Med. 7, 598–604 (2001).
Kozower, B.D. et al. Intramuscular gene transfer of interleukin-10 reduces neutrophil recruitment and ameliorates lung graft ischemia-reperfusion injury. Am. J. Transplant. 2, 837–842 (2002).
Hiratsuka, M. et al. Heat shock pretreatment protects pulmonary isografts from subsequent ischemia-reperfusion injury. J. Heart Lung Transplant. 17, 1238–1246 (1998).
Mizuta, T., Kawaguchi, A., Nakahara, K. & Kawashima, Y. Simplified rat lung transplantation using a cuff technique. J. Thorac. Cardiovasc. Surg. 97, 578–581 (1989).
Gow, A.J. et al. Immunotargeting of glucose oxidase: intracellular production of H2O2 and endothelial oxidative stress. Am. J. Physiol. 277, L271–L281 (1999).
Atochina, E.N., Muzykantov, V.R., Al-Mehdi, A.B., Danilov, S.M. & Fisher, A.B. Normoxic lung ischemia/reperfusion accelerates shedding of angiotensin converting enzyme from the pulmonary endothelium. Am. J. Respir. Crit. Care. Med. 156, 1114–1119 (1997).
Buerk, D.G. Electrochemical transducers in biology and medicine. in Biosensors: Theory and Applications 39–61 (Technomic Publishing, Lancaster, Pennsylvania, 1993).
Acknowledgements
The authors thank M. Nakada (Centocor, Malvern, Pennsylvania) for a generous gift of anti-PECAM mAb 62, R. Wiewrodt and V. Shuvaev (University of Pennsylvania) for their advice and valuable help in characterization of the conjugates size by Dynamic Light Scattering, A.P. Thomas for help in conjugate preparation and experiments with cell cultures, and D.W. Harshaw for help in experiments with perfused rat lungs. The authors also acknowledge the help of T. Tagawa and S. Kanaan (Washington University) for their support with the transplantation experiments and R. Schuessler and B.W. McKane for their statistical and laboratory assistance. S.M. is supported by a fellowship from the Fundacion Ramon Areces (Spain). The work was supported by US National Institutes of Health SCOR in Acute Lung Injury (NHLBI HL 60290, Project 4 to V.R.M. and S.M.A.), ALA Research Grant (no. RG-087-N to M.C.S.) and National Institutes of Health grant 1 R01 HL41281 (to G.A.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kozower, B., Christofidou-Solomidou, M., Sweitzer, T. et al. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat Biotechnol 21, 392–398 (2003). https://doi.org/10.1038/nbt806
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt806
This article is cited by
-
Vascular endothelial effects of collaborative binding to platelet/endothelial cell adhesion molecule-1 (PECAM-1)
Scientific Reports (2018)
-
Targeting therapeutics to endothelium: are we there yet?
Drug Delivery and Translational Research (2018)
-
Acute administration of catalase targeted to ICAM-1 attenuates neuropathology in experimental traumatic brain injury
Scientific Reports (2017)
-
Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis
Nature Medicine (2016)
-
Nanoparticle-mediated catalase delivery protects human neurons from oxidative stress
Cell Death & Disease (2013)