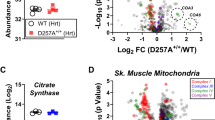
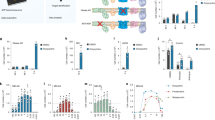
Abstract
Mitochondrial oxidative phosphorylation (OXPHOS) is under the control of both mitochondrial (mtDNA) and nuclear genomes and is central to energy homeostasis. To investigate how its function and regulation are integrated within cells, we systematically combined four cell-based assays of OXPHOS physiology with multiplexed measurements of nuclear and mtDNA gene expression across 2,490 small-molecule perturbations in cultured muscle. Mining the resulting compendium revealed, first, that protein synthesis inhibitors can decouple coordination of nuclear and mtDNA transcription; second, that a subset of HMG-CoA reductase inhibitors, combined with propranolol, can cause mitochondrial toxicity, yielding potential clues about the etiology of statin myopathy; and, third, that structurally diverse microtubule inhibitors stimulate OXPHOS transcription while suppressing reactive oxygen species, via a transcriptional mechanism involving PGC-1α and ERRα, and thus may be useful in treating age-associated degenerative disorders. Our screening compendium can be used as a discovery tool both for understanding mitochondrial biology and toxicity and for identifying novel therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
08 July 2008
In the version of this article initially published, on p.348, column 2, paragraph 2, line 7, the following sentence was incorrect: “Statins block the synthesis of cholesterol—a precursor to ubiquinone….” It should have read “Statins block the synthesis of mevalonate, a precursor not only of cholesterol but also ubiquinone, ….” The error has been corrected in the HTML and PDF versions of the article.
References
Anderson, S. et al. Sequence and organization of the human mitochondrial genome. Nature 290, 457–465 (1981).
Chance, B. & Williams, G.R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J. Biol. Chem. 217, 409–427 (1955).
DiMauro, S. & Schon, E.A. Mitochondrial respiratory-chain diseases. N. Engl. J. Med. 348, 2656–2668 (2003).
Mootha, V.K. et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Petersen, K.F. et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140–1142 (2003).
Balaban, R.S., Nemoto, S. & Finkel, T. Mitochondria, oxidants, and aging. Cell 120, 483–495 (2005).
Kelly, D.P. & Scarpulla, R.C. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 18, 357–368 (2004).
Weinstein, J.N. et al. An information-intensive approach to the molecular pharmacology of cancer. Science 275, 343–349 (1997).
Hughes, T.R. et al. Functional discovery via a compendium of expression profiles. Cell 102, 109–126 (2000).
Lamb, J. et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 313, 1929–1935 (2006).
Ramanathan, A., Wang, C. & Schreiber, S.L. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc. Natl. Acad. Sci. USA 102, 5992–5997 (2005).
Leary, S.C., Battersby, B.J., Hansford, R.G. & Moyes, C.D. Interactions between bioenergetics and mitochondrial biogenesis. Biochim. Biophys. Acta 1365, 522–530 (1998).
Dolma, S., Lessnick, S.L., Hahn, W.C. & Stockwell, B.R. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell 3, 285–296 (2003).
Smiley, S.T. et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. USA 88, 3671–3675 (1991).
Berridge, M.V. & Tan, A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 303, 474–482 (1993).
Crouch, S.P., Kozlowski, R., Slater, K.J. & Fletcher, J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 160, 81–88 (1993).
Ye, G., Metreveli, N.S., Ren, J. & Epstein, P.N. Metallothionein prevents diabetes-induced deficits in cardiomyocytes by inhibiting reactive oxygen species production. Diabetes 52, 777–783 (2003).
Stegmaier, K. et al. Gene expression–based high-throughput screening (GE-HTS) and application to leukemia differentiation. Nat. Genet. 36, 257–263 (2004).
Peck, D. et al. A method for high-throughput gene expression signature analysis. Genome Biol. 7, R61 (2006).
Kim, Y.K. et al. Relationship of stereochemical and skeletal diversity of small molecules to cellular measurement space. J. Am. Chem. Soc. 126, 14740–14745 (2004).
Franz, A.K., Dreyfuss, P.D. & Schreiber, S.L. Synthesis and cellular profiling of diverse organosilicon small molecules. J. Am. Chem. Soc. 129, 1020–1021 (2007).
Larsson, N.G. & Clayton, D.A. Molecular genetic aspects of human mitochondrial disorders. Annu. Rev. Genet. 29, 151–178 (1995).
Clayton, D.A. Transcription of the mammalian mitochondrial genome. Annu. Rev. Biochem. 53, 573–594 (1984).
Antonetti, D.A., Reynet, C. & Kahn, C.R. Increased expression of mitochondrial-encoded genes in skeletal muscle of humans with diabetes mellitus. J. Clin. Invest. 95, 1383–1388 (1995).
Huang, X. et al. Insulin-regulated mitochondrial gene expression is associated with glucose flux in human skeletal muscle. Diabetes 48, 1508–1514 (1999).
Heddi, A., Stepien, G., Benke, P.J. & Wallace, D.C. Coordinate induction of energy gene expression in tissues of mitochondrial disease patients. J. Biol. Chem. 274, 22968–22976 (1999).
Graham, D.J. et al. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. J. Am. Med. Assoc. 292, 2585–2590 (2004).
Dirks, A.J. & Jones, K.M. Statin-induced apoptosis and skeletal myopathy. Am. J. Physiol. 291, C1208–C1212 (2006).
van Vliet, A.K., Negre-Aminou, P., van Thiel, G.C., Bolhuis, P.A. & Cohen, L.H. Action of lovastatin, simvastatin, and pravastatin on sterol synthesis and their antiproliferative effect in cultured myoblasts from human striated muscle. Biochem. Pharmacol. 52, 1387–1392 (1996).
Frendin, T.J. & Swainson, C.P. Acute renal failure secondary to non-traumatic rhabdomyolysis following amoxapine overdose. N. Z. Med. J. 98, 690–691 (1985).
Blessing, W. & Walsh, J.C. Myotonia precipitated by propranolol therapy. Lancet 309, 73–74 (1977).
Davidson, B.K. Myositis associated with griseofulvin therapy. Am. Fam. Physician 52, 1277 (1995).
Delobel, P. & Pradinaud, R. Rhabdomyolysis associated with pentamidine isethionate therapy for American cutaneous leishmaniasis. J. Antimicrob. Chemother. 51, 1319–1320 (2003).
Rowinsky, E.K. et al. Phase I and pharmacologic study of paclitaxel and cisplatin with granulocyte colony-stimulating factor: neuromuscular toxicity is dose-limiting. J. Clin. Oncol. 11, 2010–2020 (1993).
Aronson, J.K. Ed. Meyler's Side Effects of Drugs 15th edn. (Elsevier Science, Burlington, Massachusetts, USA, 2006).
Bliss, C.I. The toxicity of poisons applied jointly. Ann. Appl. Biol. 26, 585–615 (1939).
Kelley, D.E., He, J., Menshikova, E.V. & Ritov, V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51, 2944–2950 (2002).
Houstis, N., Rosen, E.D. & Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440, 944–948 (2006).
Lustbader, J.W. et al. ABAD directly links Aβ to mitochondrial toxicity in Alzheimer's disease. Science 304, 448–452 (2004).
Li, W.L., Zheng, H.C., Bukuru, J. & De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 92, 1–21 (2004).
Bonawitz, N.D., Clayton, D.A. & Shadel, G.S. Initiation and beyond: multiple functions of the human mitochondrial transcription machinery. Mol. Cell 24, 813–825 (2006).
Wu, Z. et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 (1999).
Mootha, V.K. et al. Errα and Gabpa/b specify PGC-1α-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc. Natl. Acad. Sci. USA 101, 6570–6575 (2004).
Valle, I., Alvarez-Barrientos, A., Arza, E., Lamas, S. & Monsalve, M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc. Res. 66, 562–573 (2005).
Freyssenet, D., Irrcher, I., Connor, M.K., DiCarlo, M. & Hood, D.A. Calcium-regulated changes in mitochondrial phenotype in skeletal muscle cells. Am. J. Phys. 286, 1053–1061 (2004).
Caprio, S. et al. Improvement of metabolic control in diabetic patients during mebendazole administration: preliminary studies. Diabetologia 27, 52–55 (1984).
Karbowski, M. et al. Opposite effects of microtubule-stabilizing and microtubule-destabilizing drugs on biogenesis of mitochondria in mammalian cells. J. Cell Sci. 114, 281–291 (2001).
Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 199, 144–148 (1961).
Lee, P.D., Sladek, R., Greenwood, C.M. & Hudson, T.J. Control genes and variability: absence of ubiquitous reference transcripts in diverse mammalian expression studies. Genome Res. 12, 292–297 (2002).
Hieronymus, H. et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell 10, 321–330 (2006).
Acknowledgements
We thank Stephanie Norton, Jason Burbank, Mariah Eustice and Nicky Tolliday for assistance in high-throughput screening; Nathan Billings and Olga Goldberger for technical assistance; Oded Shaham, Ken Ross and Paul Clemons for computational assistance; and Joel Hirschhorn, Eric Lander and Robert Gould for thoughtful discussions and comments on the manuscript. S.L.S. and T.R.G. are Investigators of the Howard Hughes Medical Institute. V.K.M. is recipient of a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund, a Charles E. Culpeper Scholarship in Medical Science, and a Physician Scientist Early Career Award from the Howard Hughes Medical Institute. This work was supported by grants from the National Institute of Health (National Institute of Diabetes and Digestive and Kidney Diseases), the American Diabetes Association and the Richard and Susan Smith Family Foundation (V.K.M.).
Author information
Authors and Affiliations
Contributions
V.K.M. conceived of and supervised the project. B.K.W., T.K. and V.K.M. designed the experiments and analyzed the data. T.J.G., A.R. and B.K.W. carried out phenotypic screening. D.P. and T.K. carried out GE-HTS experiments. S.L.S. and T.R.G. provided guidance on chemical screening and GE-HTS, respectively, and advised on analysis. B.K.W., T.K. and V.K.M. wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Table 4 (PDF 885 kb)
Supplementary Table 1
3120 compound instances, 2490 unique compounds. (XLS 1506 kb)
Supplementary Table 2
Rank CompoundName. (XLS 18 kb)
Supplementary Table 3
ClusterID. (XLS 33 kb)
Rights and permissions
About this article
Cite this article
Wagner, B., Kitami, T., Gilbert, T. et al. Large-scale chemical dissection of mitochondrial function. Nat Biotechnol 26, 343–351 (2008). https://doi.org/10.1038/nbt1387
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1387
This article is cited by
-
The UL16 protein of HSV-1 promotes the metabolism of cell mitochondria by binding to ANT2 protein
Scientific Reports (2021)
-
SARS-CoV-2 hijacks folate and one-carbon metabolism for viral replication
Nature Communications (2021)
-
Neuronal cell-based high-throughput screen for enhancers of mitochondrial function reveals luteolin as a modulator of mitochondria-endoplasmic reticulum coupling
BMC Biology (2021)
-
Niclosamide activates the NLRP3 inflammasome by intracellular acidification and mitochondrial inhibition
Communications Biology (2019)
-
Dynamical evidence for causality between Northern Hemisphere annular mode and winter surface air temperature over Northeast Asia
Climate Dynamics (2019)