Abstract
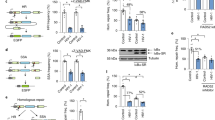
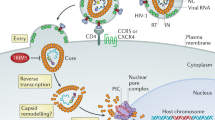
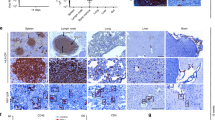
The HIV-1 RNase H can be prematurely activated by oligodeoxynucleotides targeting the highly conserved polypurine tract required for second strand DNA synthesis1,2,3,4,5. This inhibits retroviral replication in cell-free HIV particles and newly infected cells1,2,3,4. Here we extend these studies to an in vivo model of retroviral replication. Mice that are chronically infected with the spleen focus-forming virus and treated with oligodeoxynucleotides that target the polypurine tract, exhibit either transient or long-term reductions in plasma virus titer, depending on the therapeutic regimen. Treatment prior to, during or shortly after infection can delay disease progression, increase survival rates and prevent viral infection. This strategy destroys viral RNA template in virus particles in serum as well as early retroviral replication intermediates in infected cells. As it targets events common to the replication cycle of all retroviruses, this approach may be broadly applicable to retroviruses of medical and agricultural importance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Jendis, J., Strack, B., Volkmann, S., Boni, J. & Molling, K. Inhibition of replication of fresh HIV type 1 patient isolates by a polypurine tract-specific self-complementary oligodeoxynucleotide. AIDS Res. Hum. Retroviruses 12, 1161–1168 (1996).
Jendis, J., Strack, B. & Moelling, K. Inhibition of replication of drug-resistant HIV type 1 isolates by polypurine tract-specific oligodeoxynucleotide TFO A. AIDS Res. Hum. Retroviruses 14, 999–1005 (1998).
Moelling, K., Abels, S., Jendis, J., Matskevich, A. & Heinrich, J. Silencing of HIV by hairpin-loop-structured DNA oligonucleotide. FEBS Lett. 580, 3545–3550 (2006).
Matskevich, A.A., Ziogas, A., Heinrich, J., Quast, S. & Moelling, K. Short partially double-stranded oligodeoxynucleotide induces reverse transcriptase-mediated cleavage of HIV RNA and abrogates infectivity of virions. AIDS Res. Hum. Retroviruses 22, 1220–1230 (2006).
Coffin, J.M., Hughes, S.H. & Varmus,, H. Retroviruses. (Cold Spring Harbor Laboratory Press, Plainview, N.Y., 1997).
Ruscetti, S.K. Deregulation of erythropoiesis by the Friend spleen focus-forming virus. Int. J. Biochem. Cell Biol. 31, 1089–1109 (1999).
Moelling, K. Characterization of reverse transcriptase and RNase H from friend-murine leukemia virus. Virology 62, 46–59 (1974).
Tanese, N. & Goff, S.P. Domain structure of the Moloney murine leukemia virus reverse transcriptase: mutational analysis and separate expression of the DNA polymerase and RNase H activities. Proc. Natl. Acad. Sci. USA 85, 1777–1781 (1988).
Moelling, K. Reverse transcriptase and RNase H: present in a murine virus and in both subunits of an avian virus. Cold Spring Harb. Symp. Quant. Biol. 39, 969–973 (1975).
Moelling, K. et al. Association of viral reverse transcriptase with an enzyme degrading the RNA moiety of RNA-DNA hybrids. Nat. New Biol. 234, 240–243 (1971).
Tisdale, M., Schulze, T., Larder, B.A. & Moelling, K. Mutations within the RNase H domain of human immunodeficiency virus type 1 reverse transcriptase abolish virus infectivity. J. Gen. Virol. 72, 59–66 (1991).
Schulze, T., Nawrath, M. & Moelling, K. Cleavage of the HIV-1 p66 reverse transcriptase/RNase H by the p9 protease in vitro generates active p15 RNase H. Arch. Virol. 118, 179–188 (1991).
Hansen, J., Schulze, T., Mellert, W. & Moelling, K. Identification and characterization of HIV-specific RNase H by monoclonal antibody. EMBO J. 7, 239–243 (1988).
Rausch, J.W. & Le Grice, S.F. 'Binding, bending and bonding': polypurine tract-primed initiation of plus-strand DNA synthesis in human immunodeficiency virus. Int. J. Biochem. Cell Biol. 36, 1752–1766 (2004).
Jekerle, V., Kassack, M.U., Reilly, R.M., Wiese, M. & Piquette-Miller, M. Functional comparison of single- and double-stranded mdr1 antisense oligodeoxynucleotides in human ovarian cancer cell lines. J. Pharm. Pharm. Sci. 8, 516–527 (2005).
Song, J.J., Smith, S.K., Hannon, G.J. & Joshua-Tor, L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science 305, 1434–1437 (2004).
Ma, J.B. et al. Structural basis for 5′-end-specific recognition of guide RNA by the A. fulgidus Piwi protein. Nature 434, 666–670 (2005).
Yuan, Y.R. et al. Crystal structure of A. aeolicus argonaute, a site-specific DNA-guided endoribonuclease, provides insights into RISC-mediated mRNA cleavage. Mol. Cell 19, 405–419 (2005).
Moelling, K., Matskevich, A. & Jung, J.S. Relationship between retroviral replication and rna interference machineries. Cold Spring Harb. Symp. Quant. Biol. 71, 365–368 (2006).
Westerhout, E.M., ter Brake, O. & Berkhout, B. The virion-associated incoming HIV-1 RNA genome is not targeted by RNA interference. Retrovirology 3, 57–65 (2006).
Novina, C.D. et al. siRNA-directed inhibition of HIV-1 infection. Nat. Med. 8, 681–686 (2002).
Jacque, J.M., Triques, K. & Stevenson, M. Modulation of HIV-1 replication by RNA interference. Nature 418, 435–438 (2002).
Hess, C. et al. Association of a pool of HIV-1 with erythrocytes in vivo: a cohort study. Lancet 359, 2230–2234 (2002).
Bewick, V., Cheek, L. & Ball, J. Statistics review 12: survival analysis. Crit. Care 8, 389–394 (2004).
Acknowledgements
This work was in part supported by the J.B. Pendleton Charitable Trust for some animal studies with SCID mice, which we gratefully acknowledge. Some preliminary animal studies were performed by E. Operschall. We are grateful to J. Pavlovic for support of the project and for help with the animal work. We gratefully acknowledge the excellent technical assistance of Susanne Dettwiler. The animal care of P. Burger and S. Ressegatti is gratefully acknowledged. We thank E. LeClerq, Brussels, and B.A. Beutler, La Jolla, California, USA, for performing the AZT and TLR9 studies, and R. Weiss, London, for helpful discussion. We thank S. Mathur for sequencing of SFFV before and after passage.
Author information
Authors and Affiliations
Contributions
K.Matzen: animal studies; L.E.: animal studies; A.A.M. and A.N.: SFFV cell culture and in vitro studies; J.H.: in vitro studies, cleavages assays, arrangement of figures; K. Moelling: initiation and design of project, establishment of SFFV model, animal studies, writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Matzen, K., Elzaouk, L., Matskevich, A. et al. RNase H-mediated retrovirus destruction in vivo triggered by oligodeoxynucleotides. Nat Biotechnol 25, 669–674 (2007). https://doi.org/10.1038/nbt1311
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1311
This article is cited by
-
Abolishing HIV-1 infectivity using a polypurine tract-specific G-quadruplex-forming oligonucleotide
BMC Infectious Diseases (2016)
-
Short hairpin-looped oligodeoxynucleotides reduce hepatitis C virus replication
Virology Journal (2012)
-
Silencing of viral RNAs by small double-stranded siDNA
Retrovirology (2009)
-
Evolution of viruses and antiviral defense
Retrovirology (2009)
-
Short hairpin-loop-structured oligodeoxynucleotides reduce HSV-1 replication
Virology Journal (2009)