Abstract
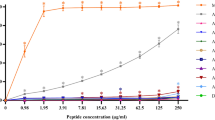
We describe peptidomimetic oligomers that show rapid, nonhemolytic, broad-spectrum bactericidal properties in mice and do not induce the emergence of resistance. The oligomers contain acyl chains, which prevent the formation of stable secondary structure. This design appears advantageous over conventional antimicrobial peptides with respect to in vivo efficacy and safety, and may provide a convenient platform for the development of peptide antibiotics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hancock, R.E. & Sahl, H.G. Nat. Biotechnol. 24, 1551–1557 (2006).
Mygind, P.H. et al. Nature 437, 975–980 (2005).
Brogden, K.A. Nat. Rev. Microbiol. 3, 238–250 (2005).
Zasloff, M. Nature 415, 389–395 (2002).
Wade, D. et al. Proc. Natl. Acad. Sci. USA 87, 4761–4765 (1990).
Yeaman, M.R. & Yount, N.Y. Pharmacol. Rev. 55, 27–55 (2003).
Feder, R., Dagan, A. & Mor, A. J. Biol. Chem. 275, 4230–4238 (2000).
Giangaspero, A., Sandri, L. & Tossi, A. Eur. J. Biochem. 268, 5589–5600 (2001).
Rotem, S., Radzishevsky, I. & Mor, A. Antimicrob. Agents Chemother. 50, 2666–2672 (2006).
Marynka, K. et al. Chem. Biol. 14, 75–85 (2007).
Leontiadou, H., Mark, A.E. & Marrink, S.J. J. Am. Chem. Soc. 128, 12156–12161 (2006).
Ge, Y. et al. Antimicrob. Agents Chemother. 43, 782–788 (1999).
Navon-Venezia, S. et al. Antimicrob. Agents Chemother. 46, 689–694 (2002).
Dartois, V. et al. Antimicrob. Agents Chemother. 49, 3302–3310 (2005).
Bradshaw, J. BioDrugs 17, 233–240 (2003).
Acknowledgements
This work was supported by The Israel Science Foundation (grant no. 387/03) and by BiolineRx (grant no. 2006992).
Author information
Authors and Affiliations
Contributions
I.S.R. synthesized reagents, performed research, analyzed data, wrote the paper; S.R. performed research (SPR and LC/MS experiments), analyzed data; D.B. performed research (in vivo blood concentrations), analyzed data; S.N.-V. performed research (in vivo toxicity and efficacy experiments), analyzed data; Y.C. designed research (in vivo toxicity and efficacy experiments), analyzed data; A.M. conceived the idea of peptide-mimetic AK oligomers, designed the OAK library, analyzed data, wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
This research was partly sponsored by BiolineRX, a drug development company supported by grants from the Israel Ministry of Trade and Industry, Chief Scientist's Office
Supplementary information
Supplementary Fig. 1
Dose-dependent binding kinetics of C12K-7α8 and MSI-78 as determined by SPR. (PDF 85 kb)
Supplementary Fig. 2
Secondary structures assessment. (PDF 83 kb)
Supplementary Fig. 3
Bacterial dissemination in-vivo. (PDF 59 kb)
Supplementary Fig. 4
Quantification of peptide concentrations in blood after i.p. administration. (PDF 43 kb)
Supplementary Fig. 5
Acute toxicity of C12K-7α8. (PDF 79 kb)
Supplementary Table 1
Physical and cytotoxic properties of representative OAKs. (PDF 14 kb)
Supplementary Table 2
Activity of C12K-7α8 against a panel of Gram-negative bacteria. (PDF 27 kb)
Supplementary Table 3
Binding properties of C12K-7α8 and MSI-78 as determined by SPR. (PDF 72 kb)
Rights and permissions
About this article
Cite this article
Radzishevsky, I., Rotem, S., Bourdetsky, D. et al. Improved antimicrobial peptides based on acyl-lysine oligomers. Nat Biotechnol 25, 657–659 (2007). https://doi.org/10.1038/nbt1309
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1309
This article is cited by
-
Sustain-release lipid-liquid crystal formulations of pexiganan against Helicobacter pylori infection: in vitro evaluation in C57BL/6 mice
BMC Pharmacology and Toxicology (2024)
-
An efflux-susceptible antibiotic-adjuvant with systemic efficacy against mouse infections
Scientific Reports (2022)
-
Three-dimensional cubic ordered mesoporous carbon with chitosan for capacitive deionization disinfection of water
Environmental Science and Pollution Research (2020)
-
BAMP-28 Antimicrobial Peptide Against Different MALDI Biotype of Carbapenam Resistant Enterobacteriaceae
International Journal of Peptide Research and Therapeutics (2019)
-
Enantiomeric glycosylated cationic block co-beta-peptides eradicate Staphylococcus aureus biofilms and antibiotic-tolerant persisters
Nature Communications (2019)