Abstract
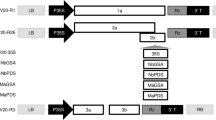
The maize dwarf mosaic virus strain B (MDMV-B) coat protein (cp) gene was cloned into a monocot expression cassette and introduced into sweet corn cell suspension cultures via particle bombardment or electroporation. Transformed cells were selected on culture media containing 300 mg/l kanamycin, and plants were regenerated. Cells from all transformed lines expressed the cp gene; and one transgenic line synthesized approximately 100–200 μg MDMV-cp per gram fresh weight. Plants regenerated from this line were challenged with a virus inoculum concentration adjusted to produce symptoms in nontransgenic controls at six days post inoculation. In growth chamber studies, the presence of the MDMV-cp provided resistance to inoculations with MDMV-A or MDMV-B and to mixed inoculations of MDMV and maize chlorotic mottle virus.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Williams, L.E. and Alexander, L.J. 1965. Maize dwarf mosaic, a new corn disease. Phytopathology 55: 802–804.
Knoke, J.K., Gingery, R.E. and Louie, R. 1992. Maize dwarf mosaic, maize chlorotic dwarf, and maize streak, p. 235–281. In: Plant Diseases of International Importance: Diseases of Cereals and Pulses. Singh, U.S., Mukhopadhyay, A.N., Kumar, J. and Chaube, H.S. (Eds.). Prentice Hall, Englewood Cliffs, NJ.
Riechmann, J.L., Lain, S. and Garcia, J.A. 1992. Highlights and prospects of potyvirus molecular biology. J. Gen. Virol. 73: 1–16.
Shaw, J.G. 1985. Early events in plant virus infections, p. 1–21. In: Molecular Plant Virology, Vol 2, Davies, J. W. (Ed.). CRC Press, Boca Raton, FL.
Osbourn, J.K., Sarkar, S. and Wilson, T.M.A. 1990. Complementation of coat protein defective TMV mutants in transgenic tobacco plants expressing TMV coat protein. Virology 179: 921–925.
McKern, N.M., Whittaker, L.A., Strike, P.M., Ford, R.E., Jensen, S.G. and Shukla, D.D. 1990. Coat protein properties indicate that maize dwarf mosaic virus-KS1 is a strain of johnsongrass mosaic virus. Phytopathology 80: 907–912.
Shukla, D.D., Tosic, M., Jilka, J., Ford, R.E., Toler, R.W. and Langham, M.A.C. 1989. Taxonomy of potyviruses infecting maize, sorghum, and sugarcane in Australia and the United States as determined by reactivities of polyclonal antibodies directed towards virus specific N-termini of coat proteins. Phytopathology 79: 223–229.
Wilson, T.M.A. 1993. Strategies to protect crop plants against viruses: Pathogen-derived resistance blossoms. Proc. Natl. Acad. Sci. USA 90: 3134–3141.
Clark, J.M., Jilka, J.M., Murry, L.M. and Scarafia, L. 1993. Virus Resistant Corn Plants. 1993. Eur. Pat. Appl. EP ♯WO 93/14210.
Murray, E.E., Lotzer, J. and Eberle, M. 1989. Codon usage in plant genes. Nucl. Acids Res. 17: 477–498.
Rodriguez-Cerezo, E., Klein, P.G. and Shaw, J.G. 1991. A determinant of disease symptom severity is located in the 3′-terminal noncoding region of the RNA of a plant virus. Proc. Natl. Acad. Sci. USA 88: 9863–9867.
Powell, P.A., Sanders, P.R., Tumer, N., Fraley, R.T. and Beachy, R.N. 1990. Protection against tobacco mosaic virus infection in transgenic plants requires accumulation of coat protein rather than coat protein RNA sequences. Virology 175: 124–130.
Klein, T.M., Fromm, M., Weissinger, A., Tomes, D., Schaaf, S., Sleten, M. and Sanford, J.C. 1988. Transfer of foreign genes into intact maize cells with high-velocity microprojectiles. Proc. Natl. Acad. Sci. USA 85: 4305–4309.
Fromm, M.E., Taylor, L.P. and Walbot, V. 1986. Stable transformation of maize after gene transfer by electroporation. Nature 319: 791–793.
Bradford, M.M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Jensen, S.G., Palomar, M.K., Ball, E.M. and Samson, R. 1985. Factors influencing virus titer in maize dwarf mosaic virus-infected sorghum. Phytopathology 75: 1132–1136.
Splittstoesser, W.E., Rest, E.B. and D'Arcy, C.J. 1990. Rapid spread of maize dwarf mosaic. Hort. Science 25: 360.
Olson, A.J., Pataky, J.K., D'Arcy, C.J. and Ford, R.E. 1990. Effects of drought stress and infection by maize dwarf mosaic virus on sweet corn. Plant Dis. 74: 147–151.
Niblett, C.L. and Claflin, L.E. 1978. Corn lethal necrosis—a new virus disease of corn in Kansas. Plant Dis. Rep. 62: 15–19.
Uyemoto, J.K. 1983. Biology and control of maize chlorotic mottle virus. Plant Dis. 67: 7–10.
Hooker, A.L. 1978. Genetics of disease resistance in maize, p. 319–322. In: Maize Breeding and Genetics, Walden, D. B. (Ed.). John Wiley & Sons, New York.
McMullen, M.D. and Louie, R. 1989. The linkage of molecular markers to a gene controlling the symptom response in maize to maize dwarf mosaic virus. Mol. Plant-Microbe Interact. 2: 309–314.
McKinney, H.H. 1929. Mosaic diseases in the Canary Islands, West Africa and Gibraltar. J. Agric. Res. 39: 557–578.
Powell-Abel, P., Nelson, R.S., De, B., Hoffman, N., Rogers, S.G., Fraley, R.T. and Beachy, R.N. 1986. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science 232: 738–743.
Gonsalves, D., Chee, P., Provvidenti, R., Seem, R. and Slightom, J.L. 1992. Comparison of coat protein-mediated and genetically derived resistance in cucumbers to infection by cucumber mosaic virus under field conditions with natural challenge inoculations by vectors. Bio/Technology 10: 1562–1570.
Beachy, R.N., Loesch-Fries, S. and Tumer, N. 1990. Coat protein-mediated resistance against virus infection. Ann. Rev. Phytopathol. 28: 451–471.
Stark, D.M. and Beachy, R.N. 1989. Protection against potyvirus infection in transgenic plants: Evidence for a broad host spectrum resistance. Bio/Technology 7: 1257–1264.
Hayakawa, T., Zhu, Y., Itoh, K., Kimura, Y., Izawa, T., Shimamoto, K. and Toriyama, S. 1993. Genetically engineered rice resistant to rice stripe virus, an insect-transmitted virus. Proc. Natl. Acad. Sci. USA 89: 9865–9869.
Namba, S., Ling, K., Gonsalves, C., Slightom, J.L. and Gonsalves, D. 1992. Protection of transgenic plants expressing the coat protein gene of watermelon mosaic virus II or zucchini yellow mosaic virus against six potyviruses. Phytopathology 82: 940–946.
Anderson, E.J., Stark, D.M., Nelson, R.M., Powell, P., Tumer, N. and Beachy, R.N. 1989. Transgenic plants that express the coat protein gene of TMV or AIMV interfere with disease development of some nonrelated viruses. Phytopathology 12: 1284–1290.
Sanders, P.R., Sammons, B., Kaniewski, W., Haley, L., Layton, J., LaVallee, B.J., Delannay, X. and Tumer, N.E. 1992. Field resistance of transgenic tomatoes expressing the tobacco mosaic virus or tomato mosaic virus coat proteins. Phytopathology 82: 683–690.
Lindbo, J.A. and Dougherty, W.G. 1992a. Untranslatable transcripts of the tobacco etch virus coat protein gene sequence can interfere with tobacco etch virus replication in transgenic plants and protoplasts. Virology 189: 725–733.
Lindbo, J.A. and Dougherty, W.G. 1992b. Pathogen-derived resistance to a potyvirus: Immune and resistant phenotypes in transgenic tobacco expressing altered forms of a potyvirus coat protein sequence. Mol. Plant-Microbe Interact. 5: 144–153.
Van der Vlugt, R.A.A., Ruiter, R.K. and Goldbach, R. 1992. Evidence for sense RNA-mediated protection to PVYN in tobacco plants transformed with the viral coat protein cistron. Plant Mol. Biol. 20: 631–639.
Wisniewski, L.A., Powell, P.A., Nelson, R.S. and Beachy, R.N. 1990. Local and systemic spread of tobacco mosaic virus (TMV) in transgenic tobacco. Plant Cell 2: 559–567.
Hilf, M.E. and Dawson, W.O. 1993. The tobamovirus capsid protein functions as a host specific determinant of long-distance movement. Virology 193: 106–114.
Register, J.C. and Beachy, R.N. 1988. Resistance to TMV in transgenic plants results from interference with an early event in the infection process. Virology 166: 524–532.
Mundry, K.W., Watkins, P.A.C., Ashfield, T., Plaskitt, K.A., Eisele-Walter, S. and Wilson, T.M.A. 1991. Complete uncoating of the 5′ leader sequence of tobacco mosaic virus RNA occurs rapidly and is required to initiate cotranslational virus disassembly in vitro. J. Gen. Virol. 72: 769–777.
Register, J.C. and Beachy, R.N. 1989. Effect of protein aggregation state on coat protein-mediated protection against tobacco mosaic virus using a transient protoplast assay system. Virology 173: 656–663.
Beachy, R.N. 1993. Virus resistance through expression of coat protein genes, p. 89–104. In: Chet, I. (Ed.). Biotechnology in Plant Disease Control. Wiley-Liss, Inc. New York.
Wilson, T.M.A. 1985. Nucleocapsid disassembly and early gene expression by positive strand RNA viruses. J. Gen. Virol. 66: 1201–1207.
Domier, L.L., Shaw, J.G. and Rhoads, R.E. 1987. Potyviral proteins share amino acid sequence homology with picorna-, como-, and caulimoviral proteins. Virology 158: 20–27.
Citovsky, V., Knorr, D., Schuster, G. and Zambryski, P. 1990. The P30 movement protein of tobacco mosaic virus is a single-strand nucleic acid binding protein. Cell 60: 637–647.
Oard, J.H., Paige, D. and Dvorak, J. 1989. Chimeric gene expression using maize intron in cultured cells of breadwheat. Plant Cell Rep. 8: 156–160.
Mascarenhas, D., Mettler, I.J., Pierce, D.A. and Lowe, H.W. 1990. Intron-mediated enhancement of heterologous gene expression in maize. Plant Mol. Biol. 15: 913–920.
Rhodes, C.A., Pierce, D.A., Mettler, I.J., Mascarenhas, D. and Detmer, J.J. 1988. Genetically transformed maize plants from protoplasts. Science 240: 204–207.
Alfinito, S.C.H., Dietrich, P.S., Murry, L.E. and Sinibaldi, R.M. 1987. A transient expression system for the assessment of plant promotors. J. Cell Biochem. 11B: 46.
Rhodes, C.A., Lowe, K.S. and Ruby, K.L. 1988. Plant regeneration from protoplasts isolated from embryogenic maize cell cultures. Bio/Technology 6: 56–60.
Murashige, T. and Skoog, F. 1963. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–479.
Maniatis, T., Fritsch, E.F. and Sambrook, J. 1982. Molecular Cloning, a Laboratory Manual. CSH Laboratory, Cold Spring Harbor, NY.
Sachs, M., Freeling, M. and Okimoto, R. 1980. Anaerobic proteins of maize. Cell 20: 761–767.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
McDonnell, R.E., Clark, R.D., Smith, W.A. and Hinchee, M.A. 1987. A simplified method for the detection of neomycin phosphotransferase II activity in transformed plant tissue. Plant Mol. Biol. Rep. 5: 380–386.
Towbin, H., Staehelin, T. and Gordon, J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76: 4350–4354.
Blake, M.S., Johnston, K.H., Russell-Jones, G.J. and Gotschlich, E.C. 1984. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on western blots. Anal. Biochem. 136: 175–179.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murry, L., Elliott, L., Capitant, S. et al. Transgenic Corn Plants Expressing MDMV Strain B Coat Protein are Resistant to Mixed Infections of Maize Dwarf Mosaic Virus and Maize Chlorotic Mottle Virus. Nat Biotechnol 11, 1559–1564 (1993). https://doi.org/10.1038/nbt1293-1559
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt1293-1559
This article is cited by
-
Maize Lethal Necrosis disease: review of molecular and genetic resistance mechanisms, socio-economic impacts, and mitigation strategies in sub-Saharan Africa
BMC Plant Biology (2022)
-
Protective role of S-methylmethionine-salicylate in maize plants infected with Maize dwarf mosaic virus
European Journal of Plant Pathology (2017)
-
RNA interference-mediated resistance to maize dwarf mosaic virus
Plant Cell, Tissue and Organ Culture (PCTOC) (2013)