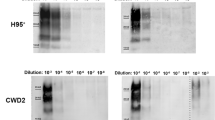
Abstract
Prion diseases are caused by propagation of misfolded forms of the normal cellular prion protein PrPC, such as PrPBSE in bovine spongiform encephalopathy (BSE) in cattle and PrPCJD in Creutzfeldt-Jakob disease (CJD) in humans1. Disruption of PrPC expression in mice, a species that does not naturally contract prion diseases, results in no apparent developmental abnormalities2,3,4,5. However, the impact of ablating PrPC function in natural host species of prion diseases is unknown. Here we report the generation and characterization of PrPC-deficient cattle produced by a sequential gene-targeting system6. At over 20 months of age, the cattle are clinically, physiologically, histopathologically, immunologically and reproductively normal. Brain tissue homogenates are resistant to prion propagation in vitro as assessed by protein misfolding cyclic amplification7. PrPC-deficient cattle may be a useful model for prion research and could provide industrial bovine products free of prion proteins.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Prusiner, S.B. Detecting mad cow disease. Sci. Am. 291, 86–93 (2004).
Bueler, H. et al. Normal development and behavior of mice lacking the neural cell-surface PrP protein. Nature 356, 577–582 (1992).
Manson, J.C. et al. 129/Ola mice carrying a null mutation in PrP that abolishes mRNA production are developmentally normal. Mol. Neurobiol. 8, 121–127 (1994).
Prusiner, S.B. et al. Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies. Proc. Natl. Acad. Sci. USA 90, 10608–10612 (1993).
Bueler, H. et al. Mice devoid of PrP are resistant to scapie. Cell 73, 1339–1347 (1993).
Kuroiwa, Y. et al. Sequential targeting of the genes encoding immunoglobulin-μ and prion protein in cattle. Nat. Genet. 36, 775–780 (2004).
Saborio, G.P., Permmane, B. & Soto, C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411, 810–813 (2001).
Berthelin, B.C . Scrapie in Sheep: Clinical Features and Differential Diagnostic, VIth International Workshop on the Diagnosis of Spongiform Encephalopathies, Weybridge, UK, November 25–29, 2002 (Veterinary Laboratories Agency, Weybridge, UK, 2002).
Vargas, F. et al. Detection and clinical evolution of scrapie in sheep using third eyelid biopsy. J. Vet. Intern. Med. 20, 187–193 (2006).
Chesebro, B. Prion protein and the transmissible spongiform encephalopathy diseases. Neuron 24, 503–506 (1999).
Prusiner, S.B. Prions. Proc. Natl. Acad. Sci. USA 95, 13363–13383 (1998).
Mabbott, N. & Turner, M. Prions and the blood and immune systems. Haematologica 90, 542–548 (2005).
Aguzzi, A. Prion disease, blood and the immune system: concerns and realty. Haematologica 85, 3–10 (2000).
Bainbridge, J. & Walker, K.B. The normal cellular form of prion protein modulates T cell responses. Immunol. Lett. 96, 147–150 (2005).
Mabbott, N.A., Brown, K.L., Manson, J. & Bruce, M.E. T-lymphocyte activation and the cellular form of the prion protein. Immunology 92, 161–165 (1997).
Castilla, J., Saa, P., Hetz, C. & Soto, C. In vitro generation of infectious scrapie prions. Cell 121, 195–206 (2005).
Saa, P., Castilla, J. & Soto, C. Cyclic amplification of protein misfolding and aggregation. Methods Mol. Biol. 299, 53–65 (2005).
Castilla, J., Saa, P. & Soto, C. in Methods and Tools in Bioscience and Medicine (eds. Lehmann, S. & Grassi, J.) 198–213, (Birkhauser Verlag, Basel, 2004).
Deleault, N.R., Lucassen, R.W. & Supattapone, S. RNA molecules stimulate prion protein conversion. Nature 425, 717–720 (2003).
Wong, C. et al. Sulfated glycans and elevated temperature stimulate PrPSc-dependent cell-free formation of protease-resistant prion protein. EMBO J. 20, 377–386 (2001).
Seabury, C.M. et al. Prion protein gene (PRNP) variants and evidence for strong purifying selection in functionally important regions of bovine exon 3. Proc. Natl. Acad. Sci. USA 101, 15142–15147 (2004).
Castilla, J., Saa, P. & Soto, C. Detection of prions in blood. Nat. Med. 11, 982–985 (2005).
Sakaguchi, S. et al. Loss of cerebellar Purkinje cells in aged mice homozygous for a disrupted PrP gene. Nature 380, 528–531 (1996).
Moore, R.C. et al. Ataxia in prion protein (PrP)-deficient mice is associated with upregulation of the novel PrP-like protein doppel. J. Mol. Biol. 292, 797–817 (1999).
Rossi, D. et al. Onset of ataxia and Purkinje cell loss in PrP null mice inversely correlated with Dpl level in brain. EMBO J. 20, 694–702 (2001).
Silverman, G.L. et al. Doppel is an N-glycosylated, glycosylphosphatidylinositol-anchored protein. Expression in testis and ectopic production in the brains of Prnp0/0 mice predisposed to Purkinje cell loss. J. Biol. Chem. 275, 26834–26841 (2000).
Weissmann, C., Enari, M., Klohn, P.C., Rossi, D. & Flechsig, E. Transmission of prions. Proc. Natl. Acad. Sci. USA 99 Suppl 4, 16378–16383 (2002).
Li, A. et al. Identification of a novel gene encoding a PrP-like protein expressed as chimeric transcripts fused to PrP exon 1/2 in ataxic mouse line with a disrupted PrP gene. Cell. Mol. Neurobiol. 20, 553–567 (2000).
Collinge, J. et al. Prion protein is necessary for normal synaptic function. Nature 370, 295–297 (1994).
Mallucci, G.R. et al. Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration. EMBO J. 21, 202–210 (2002).
Tobler, I., Deboer, T. & Fischer, M. Sleep and sleep regulation in normal and prion protein-deficient mice. J. Neurosci. 17, 1869–1879 (1997).
Tobler, I. et al. Altered circadian activity rhythms and sleep in mice devoid of prion protein. Nature 380, 639–642 (1996).
Lledo, P.M. et al. Mice deficient for prion protein exhibit normal neuronal excitability and synaptic transmission in the hippocampus. Proc. Natl. Acad. Sci. USA 93, 2403–2407 (1996).
Criado, J.R. et al. Mice devoid of prion protein have cognitive deficits that are rescued by reconstitution of PrP in neurons. Neurobiol. Dis. 19, 255–265 (2005).
Shmerling, D. et al. Expression of amino-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell 93, 203–214 (1998).
Biacabe, A.G., Laplanche, J.L., Ryder, S. & Baron, T. Distinct molecular phenotypes in bovine prion diseases. EMBO Rep. 5, 110–115 (2003).
Yamakawa, Y. et al. Atypical Proteinase K-resistant prion protein (PrPres) observed in an apparently healthy 23-month-old Holstein steer. Jpn. J. Infect. Dis. 56, 221–222 (2003).
Casalone, C. et al. Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease. Proc. Natl. Acad. Sci. USA 101, 3065–3070 (2004).
Acknowledgements
We thank the team at Transova Genetics for their efforts in embryo transfer and Todd Stahl, Rebecca Cuperus, Maria Diaz, Cliff Mazour for their assistance in calf delivery and care. We thank Melanie Nichols, Jason Griffin, Melissa Bien, Molly Ahlers, Rachael Paulson, Sarah Viet, Cassandra Voss for their assistance in gene targeting, cell culture and embryonic cloning. We thank Kevin Hassall for an additional western blotting. We also thank Ralph Kubo and Tomoyuki Tahara for their useful comments on the immunological study. Soto is part of the European Community project TSELAB and was supported in part by National Institutes of Health grants NS050349 and NS049173.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Some authors are employees of Hematech Inc., Gemini Science Inc. or Kirin Co., Ltd. These companies funded the work.
Supplementary information
Supplementary Fig. 1
Fertility of PRNP−/− bovine sperm.
Supplementary Fig. 2
PRNP−/−IGHM−/− double knockout (DKO) cattle.
Supplementary Table 1
Serum chemistry values for PRNP cattle.
Supplementary Table 2
Hematology values for PRNP−/− and control cattle.
Supplementary Table 3
Comparison of lymphocyte populations between PRNP−/− and wild-type cattle.
Supplementary Table 4
Blastocyst development in IVF between sperm from the PRNP−/− bulls and oocytes from wild-type cows.
Supplementary Table 5
Blastocyst development from PRNP−/−fibroblast cell lines.
Rights and permissions
About this article
Cite this article
Richt, J., Kasinathan, P., Hamir, A. et al. Production of cattle lacking prion protein. Nat Biotechnol 25, 132–138 (2007). https://doi.org/10.1038/nbt1271
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1271
This article is cited by
-
Classical bovine spongiform encephalopathy and chronic wasting disease: two sides of the prion coin
Animal Diseases (2023)
-
Mechanisms of prion-induced toxicity
Cell and Tissue Research (2023)
-
Collagen-Based Medical Devices for Regenerative Medicine and Tissue Engineering
Applied Biochemistry and Biotechnology (2023)
-
Advancing genome editing to improve the sustainability and resiliency of animal agriculture
CABI Agriculture and Bioscience (2022)
-
Towards progressive regulatory approaches for agricultural applications of animal biotechnology
Transgenic Research (2022)