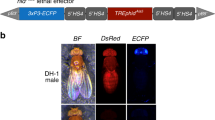
Abstract
Methods involving the release of transgenic insects in the field hold great promise for controlling vector-borne diseases and agricultural pests. Insect transformation depends on nonautonomous transposable elements as gene vectors. The resulting insertions are stable in the absence of suitable transposase, however, such absence cannot always be guaranteed. We describe a method for post-integration elimination of all transposon sequences in the pest insect Medfly, Ceratitis capitata. The resulting insertions lack transposon sequences and are therefore impervious to transposase activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Li, X. et al. Insect Mol. Biol. 14, 17–30 (2005).
Wimmer, E. Nat. Rev. Genet. 4, 225–232 (2003).
Atkinson, P., Pinkerton, A. & O'Brochta, D . Annu. Rev. Entomol. 46, 317–346 (2001).
Alphey, L. Insect Biochem. Mol. Biol. 32, 1243–1247 (2002).
Alphey, L. et al. Science 298, 119–121 (2002).
Olson, K.E., Alphey, L., Carlson, J. & James, A.A. in Bridging Laboratory and Field Research for Genetic Control of Disease Vectors, vol. 11 (eds. Knols, B.G.J. & Louis, C.) 210 (Springer, Heidelberg, 2006).
Handler, A . Insect Biochem. Mol. Biol. 34, 121–130 (2004).
Hoy, M. in Insect transgenesis: methods and applications (eds. James, A. & Handler, A.) 335–367 (CRC Press, Boca Raton, 2000).
Handler, A., Zimowska, G. & Horn, C. Nat. Biotechnol. 22, 1150–1154 (2004).
Li, X., Lobo, N., Bauser, C. & Fraser, M. Mol. Genet. Genomics 266, 190–198 (2001).
Berg, C. & Spradling, A. Genetics 127, 515–524 (1991).
Horn, C. & Handler, A. . Proc. Natl. Acad. Sci. USA 102, 12483–12488 (2005).
Oberstein, A., Pare, A., Kaplan, L. & Small, S. Nat. Methods 2, 583–585 (2005).
Nimmo, D., Alphey, L., Meredith, J. & Eggleston, P. Insect Mol. Biol. 15, 129–136 (2006).
Shagin, D.A. et al. Mol. Biol. Evol. 21, 841–850 (2004).
Acknowledgements
We thank Karen Clifton and staff at Oxitec for technical assistance, Nadia Pantic and Peng Gong for help at early stages of the project, Pedro Rendón for the Toliman Medfly strain and Tassos Mintzas, Sinead O'Connell and Derric Nimmo for advice and critical review of the manuscript. This work was funded in part by the UK Biotechnology and Biological Sciences Research Council.
Author information
Authors and Affiliations
Contributions
T.H.D. and G.C.C. contributed equally to this work. T.H.D., C.E.P. and L.J. designed and constructed the DNA constructs and assisted with the molecular analysis of transgenics. G.C.C. and K.C.C. created the transgenic Medflies and conducted most of the phenotypic and molecular analysis. N.I.M., M.J.E. and G.F. performed preliminary experiments and developed the marker system. L.A. conceived and supervised the project and wrote the paper with G.C.C. All authors discussed the results and commented on the manuscript.
Note: Supplementary information is available on the Nature Biotechnology website.
Corresponding author
Ethics declarations
Competing interests
Those authors affiliated with Oxitec Ltd. are employees or students of this company. Oxitec supplied salary and other support for the research program. Also, some of these authors have shares or share options in Oxitec Ltd. Both Oxitec Ltd. and Oxford University have one or more patents or patent applications related to the subject of this paper.
Supplementary information
Supplementary Fig. 1
piggyBac-based constructs used in this paper. (PDF 121 kb)
Supplementary Fig. 2
Sequence and location of primers used to analyse insertions. (PDF 115 kb)
Supplementary Fig. 3
Expression of CopGreen, PhiYFP and J-Red fluorescent markers. (PDF 125 kb)
Supplementary Table 1
Post-integration removal of piggyBac ends. (PDF 97 kb)
Supplementary Table 2
Mobilization rates of an X-linked composite insertion. (PDF 94 kb)
Supplementary Table 3
Mobilization rates of integrated transposons. (PDF 116 kb)
Rights and permissions
About this article
Cite this article
Dafa'alla, T., Condon, G., Condon, K. et al. Transposon-free insertions for insect genetic engineering. Nat Biotechnol 24, 820–821 (2006). https://doi.org/10.1038/nbt1221
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1221
This article is cited by
-
Unpredictable recombination of PB transposon in Silkworm: a potential risk
Molecular Genetics and Genomics (2021)
-
An early female lethal system of the New World screwworm, Cochliomyia hominivorax, for biotechnology-enhanced SIT
BMC Genetics (2020)
-
Highly efficient DNA-free gene disruption in the agricultural pest Ceratitis capitata by CRISPR-Cas9 ribonucleoprotein complexes
Scientific Reports (2017)
-
The whole genome sequence of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann), reveals insights into the biology and adaptive evolution of a highly invasive pest species
Genome Biology (2016)
-
An efficient strategy for producing a stable, replaceable, highly efficient transgene expression system in silkworm, Bombyx mori
Scientific Reports (2015)