Abstract
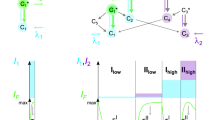
Dual-color fluorescence cross-correlation spectroscopy (FCCS) is a promising technique for quantifying protein-protein interactions1,2,3,4,5. In this technique, two different fluorescent labels are excited and detected simultaneously within a common measurement volume. Difficulties in aligning two laser lines and emission crossover between the two fluorophores, however, make this technique complex. To overcome these limitations, we developed a fluorescent protein with a large Stokes shift. This protein, named Keima, absorbs and emits light maximally at 440 nm and 620 nm, respectively. Combining a monomeric version of Keima with cyan fluorescent protein allowed dual-color FCCS with a single 458-nm laser line and complete separation of the fluorescent protein emissions. This FCCS approach enabled sensitive detection of proteolysis by caspase-3 and the association of calmodulin with calmodulin-dependent enzymes. In addition, Keima and a spectral variant that emits maximally at 570 nm might facilitate simultaneous multicolor imaging with single-wavelength excitation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kettling, U., Koltermann, A., Schwille, P. & Eigen, M. Real-time enzyme kinetics monitored by dual-color fluorescence cross-correlation spectroscopy. Proc. Natl. Acad. Sci. USA 95, 1416–1420 (1998).
Weidemann, T., Wachsmuth, M., Tewes, M., Rippe, K. & Langowski, J. Analysis of ligand binding by two-colour fluorescence cross-correlation spectroscopy. Single Mol. 3, 49–61 (2002).
Kim, S.A. & Schwille, P. Intracellular applications of fluorescence correlation spectroscopy: prospects for neuroscience. Curr. Opin. Neurobiol. 13, 583–590 (2003).
Saito, K., Wada, I., Tamura, M. & Kinjo, M. Direct detection of caspase-3 activation in single live cells by cross-correlation analysis. Biochem. Biophys. Res. Commun. 324, 849–854 (2004).
Kohl, T., Haustein, E. & Schwille, P. Determining protease activity in vivo by fluorescence cross-correlation analysis. Biophys. J. 89, 2770–2782 (2005).
Hwang, L.C. & Wohland, T. Single wavelength excitation fluorescence cross-correlation spectroscopy with spectrally similar fluorophores: Resolution for binding studies. J. Chem. Phys. 122, 114708 (1–11) (2005).
Martin, B.R., Giepmans, B.N., Adams, S.R. & Tsien, R.Y. Mammalian cell-based optimization of the biarsenical-binding tetracysteine motif for improved fluorescence and affinity. Nat. Biotechnol. 23, 1308–1314 (2005).
Helmchen, F. & Denk, W. New developments in multiphoton microscopy. Curr. Opin. Neurobiol. 12, 593–601 (2002).
Heinze, K.G., Koltermann, A. & Schwille, P. Simultaneous two-photon excitation of distinct labels for dual-color fluorescence crosscorrelation analysis. Proc. Natl. Acad. Sci. USA 97, 10377–10382 (2000).
Kohl, T., Heinze, K.G., Kuhlemann, R., Koltermann, A. & Schwille, P. A protease assay for two-photon crosscorrelation and FRET analysis based solely on fluorescent proteins. Proc. Natl. Acad. Sci. USA 99, 12161–12166 (2002).
Heinze, K.G., Rarbach, M., Jahnz, M. & Schwille, P. Two-photon fluorescence coincidence analysis: rapid measurements of enzyme kinetics. Biophys. J. 83, 1671–1681 (2002).
Kim, S.A., Heinze, K.G., Waxham, M.N. & Schwille, P. Intracellular calmodulin availability accessed with two-photon cross-correlation. Proc. Natl. Acad. Sci. USA 101, 105–110 (2004).
Patterson, G.H. & Piston, D.W. Photobleaching in two-photon excitation microscopy. Biophys. J. 78, 2159–2162 (2000).
Chen, T.S., Zeng, S.Q., Luo, Q.M., Zhang, Z.H. & Zhou, W. High-order photobleaching of green fluorescent protein inside live cells in two-photon excitation microscopy. Biophys. Biochem. Res. Commun. 291, 1272–1275 (2002).
Matz, M.V., Lukyanov, K.A. & Lukyanov, S.A. Family of the green fluorescent protein: journey to the end of the rainbow. Bioessays 24, 953–959 (2002).
Labas, Y.A. et al. Diversity and evolution of the green fluorescent protein family. Proc. Natl. Acad. Sci. USA 99, 4256–4261 (2002).
Tsutsui, H., Karasawa, S., Shimizu, H., Nukina, N. & Miyawaki, A. Semi-rational engineering of a coral fluorescent protein into an efficient highlighter. EMBO Rep. 6, 233–238 (2005).
Tsien, R.Y. The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544 (1998).
Campbell, R.E. et al. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 7877–7882 (2002).
Crivici, A. & Ikura, M. Molecular and structural basis of target recognition by calmodulin. Annu. Rev. Biophys. Biomol. Struct. 24, 85–116 (1995).
Nagai, T., Yamada, S., Tominaga, T., Ichikawa, M. & Miyawaki, A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA 101, 10554–10559 (2004).
Karasawa, S., Araki, T., Nagai, T., Mizuno, H. & Miyawaki, A. Cyan-emitting and orange-emitting fluorescent proteins as a donor/acceptor pair for fluorescence resonance energy transfer. Biochem. J. 381, 307–312 (2004).
Karasawa, S., Araki, T., Yamamoto-Hino, M. & Miyawaki, A. A green-emitting fluorescent protein from Galaxeidae coral and its monomeric version for use in fluorescent labeling. J. Biol. Chem. 278, 34167–34171 (2003).
Sawano, A. & Miyawaki, A. Directed evolution of green fluorescent protein by a new versatile PCR strategy for site-directed and semi-random mutagenesis. Nucleic Acids Res. 28, E78 (2000).
Arscott, P.L. et al. Fas (CD95) expression is up-regulated on papillary thyroid carcinoma. J. Clin. Endocrinol. Metab. 84, 4246–4252 (1999).
Llopis, J., McCaffery, J.M., Miyawaki, A., Farquhar, M.G. & Tsien, R.Y. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. USA 95, 6803–6808 (1998).
Sato, S. et al. Aberrant tau phosphorylation by glycogen synthase kinase-3b and JNK3 induces oligomeric tau fibrils in COS-7 cells. J. Biol. Chem. 277, 2060–2065 (2002).
Sawano, A., Hama, H., Saito, N. & Miyawaki, A. Multicolor imaging of Ca2+ and protein kinase C signals using novel epifluorescence microscopy. Biophys. J. 82, 1076–1085 (2002).
Mochizuki, N. et al. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature 411, 1065–1068 (2001).
Miyawaki, A. et al. Fluorescent indicator for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388, 882–887 (1997).
Acknowledgements
The authors would like to thank K. Iwao and S. Hosaka at the Akajima Marine Science Laboratory for acquiring the stony coral animals, Y. Isogai for assistance with analytical centrifugation, F. Ishidate, K. Weisshart, B. Zimmerman, Y. Hasegawa for assistance with FCCS measurements and spectral imaging, and K. Ishihara, H. Watanabe, T. Fukano, and M. Hirano for assistance with multi-color imaging and fluorescence lifetime measurements. This work was partly supported by grants from Japan MEXT Grant-in-Aid for Scientific Research on priority areas, NEDO (the New Energy and Industrial Technology Development Organization), HFSP (the Human Frontier Science Program), and RIKEN Strategic Research Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S.K. and T.A. get salaries from Amalgaam and Medical Biological Laboratories, which will sell the products presented in this paper.
Supplementary information
Supplementary Fig. 1
An amino-acid sequence alignment of DsRed with #20, #20-9115, tKeima, dKeima, mKeima, and dKeima570. (PDF 194 kb)
Supplementary Fig. 2
Equilibrium radial absorbance profiles obtained by analytical ultracentrifugation for Keima variants. (PDF 288 kb)
Supplementary Fig. 3
Quantitative performance of FCCS using an mKeima-CFP pair. (PDF 262 kb)
Supplementary Fig. 4
Detection of the Ca2+-dependent association between CaM and CaMKI with the SL-FCCS technique using mKeima and CFP. (PDF 219 kb)
Supplementary Fig. 5
Single laser wavelength (458 nm) excitation FCCS to monitor the Ca2+-dependent association between CaM and M13 in a living HeLa cell, which was expressing mCFP-CaM and M13-mKeima. (PDF 251 kb)
Supplementary Fig. 6
Simultaneous imaging of [Ca2+]c and mitochondrial morphology. (PDF 937 kb)
Supplementary Fig. 7
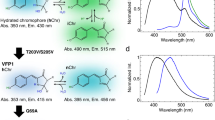
Excitation (broken lines) and emission (solid lines) spectra of dKeima and dKeima570. (PDF 215 kb)
Supplementary Table 1
Spectral characteristics of CFP, Keima, dKeima, mKeima, and dKeima570. (PDF 85 kb)
Rights and permissions
About this article
Cite this article
Kogure, T., Karasawa, S., Araki, T. et al. A fluorescent variant of a protein from the stony coral Montipora facilitates dual-color single-laser fluorescence cross-correlation spectroscopy. Nat Biotechnol 24, 577–581 (2006). https://doi.org/10.1038/nbt1207
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1207
This article is cited by
-
Large Stokes shift fluorescent RNAs for dual-emission fluorescence and bioluminescence imaging in live cells
Nature Methods (2023)
-
A highly photostable and bright green fluorescent protein
Nature Biotechnology (2022)
-
Structure- and mechanism-guided design of single fluorescent protein-based biosensors
Nature Chemical Biology (2021)
-
Super stable CsPbBr3@SiO2 tumor imaging reagent by stress-response encapsulation
Nano Research (2020)