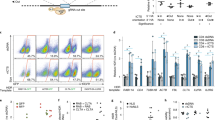
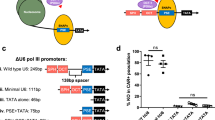
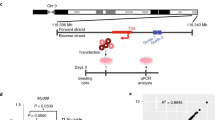
Abstract
RNA interference occurs when cytoplasmic small interfering RNAs (siRNAs) enter the RNA-induced silencing complex and one strand guides cleavage of the target RNA by the Argonaute 2 protein1,2,3. A significant concern when applying siRNAs or expressing small hairpin RNAs (shRNAs) in human cells is activation of the interferon (IFN) response4,5,6,7,8,9,10. Synthetic siRNAs harboring certain motifs can induce an immune response when delivered to mouse and human immune cells such as peripheral blood mononuclear cells, monocytes, plasmacytoid dendritic cells (pDCs) and nonplasmacytoid dendritic cells (mDCs)11,12,13. In the present study we have tested the immunostimulatory effects of lipid-delivered siRNAs versus Pol III promoter–expressed shRNAs in primary CD34+ progenitor–derived hematopoietic cells. We show that in this system, lipid-delivered siRNAs are potent inducers of IFNα and type I IFN gene expression, whereas the same sequences when expressed endogenously are nonimmunostimulatory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Meister, G. & Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 431, 343–349 (2004).
Ma, J.B. et al. Structural basis for 5′-end-specific recognition of guide RNA by the A. fulgidus Piwi protein. Nature 434, 666–670 (2005).
Parker, J.S., Roe, S.M. & Barford, D. Structural insights into mRNA recognition from a PIWI domain-siRNA guide complex. Nature 434, 663–666 (2005).
Yoneyama, M. et al. The RNA helicase RIG-1 has an essential function in double-stranded RNA induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Elbashir, S.M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Sledz, C.A., Holko, M., de Veer, M.J., Silverman, R.H. & Williams, B.R.G. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 5, 834–883 (2003).
Bridge, A.J., Pebernard, S., Ducraux, A., Nicoulaz, A.L. & Iggo, R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat. Genet. 34, 263–264 (2003).
Kim, D.H. et al. Interferon induction by siRNAs and ssRNAs synthesized by phage polymerase. Nat. Biotechnol. 22, 321–325 (2004).
Kariko, K. et al. Exogenous siRNA mediates sequence-independent gene suppression by signaling through Toll-like receptor 3. Cells Tissues Organs 177, 132–138 (2004).
Pebernard, S. & Iggo, R. Determinants of interferon-stimulated gene induction by RNAi vectors. Differentiation 72, 103–111 (2004).
Sioud, M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J. Mol. Biol. 348, 1079–1090 (2005).
Hornung, V. et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 11, 263–270 (2005).
Judge, A.D. et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 23, 457–462 (2005).
Wang, L. et al. Noncoding RNA danger motifs bridge innate and adaptive immunity and are potent adjuvants for vaccination. J. Clin. Invest. 110, 1175–1184 (2002).
Heil, F. et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
Li, M. et al. Long-term inhibition of HIV-1 infection in primary hematopoietic cells by lentiviral vector delivery of a triple combination of anti-HIV shRNA, anti-CCR5 ribozyme, and a nucleolar-localizing TAR decoy. Mol. Ther. 12, 900–909 (2005).
Diebold, S.S. et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424, 324–328 (2003).
Giron-Michel, J. et al. Direct signal transduction via functional interferon-αβ receptors in CD34+ hematopoietic stem cells. Leukemia 16, 1135–1142 (2002).
Kim, S., Choi, Y., Bazer, W. & Spencer, T.E. Identification of genes in the ovine endometrium regulated by interferon tau independent of signal transducer and activator of transcription 1. Endocrinology 144, 5203–5214 (2003).
Vandesompele, J. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, Research0034 (2002).
MacQuillan, G.C., Mamotte, C., Reed, W.D., Jeffrey, G.P. & Allan, J.E. Upregulation of endogenous intrahepatic interferon stimulated genes during chronic hepatitis C virus infection. J. Med. Virol. 70, 219–227 (2003).
Lee, N.S. et al. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat. Biotechnol. 20, 500–505 (2002).
Acknowledgements
The authors would like to thank Lucy Brown and Claude Yosballa at the COH FACS Facility for FACS assays and analysis. This work was supported by National Institutes of Health grants AI29329, AI42552, HL07470 and AI061389.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
CD34+ progenitor derived immune cells are a mixed population of monocytes and dendritic cells. (PDF 83 kb)
Supplementary Fig. 2
Schematic of HIV-7 vector used to transduce CD34+ progenitor cells with therapeutic RNAs. (PDF 94 kb)
Supplementary Fig. 3
Inhibition of HIV-1 replication in CD34+ cells expressing anti-HIV RNAs. (PDF 60 kb)
Supplementary Fig. 4
CD34+ progenitors expressing GFP only or shRNA or a triple cassette differentiate to monocytes to similar levels. (PDF 91 kb)
Supplementary Fig. 5
Differentiation of control or stably transduced CD34+ progenitor derived immune cells expressing GFP, shII or the triple construct produces a mixed population of monocytes and plasmacytoid dendritic cells. (PDF 75 kb)
Rights and permissions
About this article
Cite this article
Robbins, M., Li, M., Leung, I. et al. Stable expression of shRNAs in human CD34+ progenitor cells can avoid induction of interferon responses to siRNAs in vitro. Nat Biotechnol 24, 566–571 (2006). https://doi.org/10.1038/nbt1206
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1206
This article is cited by
-
A Novel Approach to Block HIV-1 Coreceptor CXCR4 in Non-toxic Manner
Molecular Biotechnology (2014)
-
Viral infection resistance conferred on mice by siRNA transgenesis
Transgenic Research (2013)
-
Progress Toward In Vivo Use of siRNAs-II
Molecular Therapy (2012)
-
Current progress in the development of RNAi-based therapeutics for HIV-1
Gene Therapy (2011)