Abstract
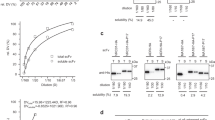
The combination of single-chain Fv-fragments (scFv) with a C-terminal, flexible linking region followed by a designed or natural dimerization domain provides a versatile system for targeted association of functional fragments in the periplasmic space of Escherichia coli. For homodimerization in vivo, two scFv fragments with a C-terminal hinge followed by a helix-turn-helix motif form “miniantibodies” with significantly higher avidity than in the case of leucine zipper containing constructs. The favorable design probably results in an antiparallel four-helix bundle and brings the homodimer to the same avidity as the whole IgA antibody, from which the binding site was taken. The molecular weight of the bivalent miniantibody is almost the same as that of a monovalent Fab fragment. We report here a high-cell density fermentation of E. coli producing these miniantibodies and a work-up procedure suitable for large scale production. Without any need of subsequent chemical coupling in vitro, approximately 200 mg/l of functional dimeric miniantibodies can be directly obtained from the E. coli culture.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Pack, P. and Plückthun, A. 1992. Miniantibodies: use of amphipathic helices to produce functional, flexibly linked dimeric Fv fragments with high avidity in Escherichia coli . Biochemistry 31: 1579–1584.
Crothers, D.M. and Metzger, H. 1972. The influence of polyvalency on the binding properties of antibodies. Immunochemistry 9: 341–357.
Karush, F. 1978. The affinity of antibody: range, variability, and the role of multivalence, pp. 85–116. In: Immunoglobulins. Litman, G. W. and Good, R. A. (Eds.). Plenum Publishing Corp., NY and London.
Glockshuber, R., Malia, M., Pfitzinger, I. and Plückthun, A. 1990. A comparison of strategies to stabilize immunoglobulin Fv-fragments. Biochemistry 29: 1362–1367.
Bird, R.E., Hardman, K.D., Jacobson, J.W., Johnson, S., Kaufman, B.M., Lee, S.-M., Lee, T., Pope, S.H., Riordan, G.S. and Whitlow, M. 1988. Single-chain antigen-binding proteins. Science 242: 423–426.
Huston, J.S., Levinson, D., Mudgett-Hunter, M., Tai, M.-S., Novotny, J., Margolies, M.N., Ridge, R.J., Bruccoleri, R.E., Haber, E., Crea, R. and Oppermann, H. 1988. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli . Proc. Natl. Acad. Sci. USA 85: 5879–5883.
O'Shea, E.K., Klemm, J.D., Kim, P.S. and Alber, T. 1991. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science 254: 539–545.
O'Shea, E.K., Rutkowski, R. and Kim, P.S. 1989. Evidence that the leucine zipper is a coiled coil. Science 243: 538–542.
Eisenberg, D., Wilcox, W., Eshita, S.M., Pryciak, P.M., Ho, S.P. and DeGrado, W.F. 1986. The design, synthesis, and crystallization of an alpha-helical peptide. Proteins 1: 16–22.
Ho, S.P. and DeGrado, W.F. 1987. Design of a 4-helix bundle protein: synthesis of peptides which self-associate into a helical protein. J. Am. Chem. Soc. 109: 6751–6758.
Glockshuber, R., Schmidt, T. and Plückthun, A. 1992. The disulfide bonds in antibody variable domains: effects on stability, folding in vitro, and functional expression in Escherichia coli . Biochemistry 31: 1270–1279.
Satow, Y., Cohen, G.H., Padlan, E.A. and Davies, D.R. 1986. Phosphocholine binding immunoglobulin Fab McPC603. An X-ray diffraction study at 2.7Å. J. Mol. Biol. 190: 593–604.
Skerra, A. and Plückthun, A. 1988. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli . Science 240: 1038–1041.
Plückthun, A. and Skerra, A. 1989. Expression of functional Fv and Fab fragments in Escherichia coli . Meth. Enzymol. 178: 497–515.
Pack, P., Knappik, A., Krebber, C. and Plückthun, A. 1992. Mono- and bivalent antibody fragments produced in E. coli : binding properties and folding in vivo, pp. 10–13. In: ACS Conference Proceedings Series, Harnessing Biotechnology for the 21st Century. Ladisch, M. R. and Bose, A. (Eds.) ACS, Washington, DC.
Wülfing, C. and Plückthun, A. 1993. A versatile and highly repressible E. coli expression system based on invertible promoters. Gene. In press
Riesenberg, D. 1991. High-cell-density cultivation of Escherichia coli . Current Opinion in Biotechnol. 2: 380–384.
Metzger, H., Chesebro, B., Hadler, N.M., Lee, J. and Otchin, N. 1971. Modification of immunoglobulin combining sites, pp. 253–267. In: Progress in immunology: Proceedings of the 1st Congress of Immunology. Amos, B. (Ed.). Academic Press, New York.
Blondel, A. and Bedouelle, H. 1991. Engineering the quaternary structure of an exported protein with a leucine zipper. Prot. Engineering 4: 457–461.
Hu, J.C., O'Shea, E.K., Kim, P.S. and Sauer, R.T. 1990. Sequence requirements for coiled coils: analysis with λ-repressor-GCN4 leucine zipper fusions. Science 250: 1400–1403.
Kostelny, S.A., Cole, M.S. and Tso, Y.J. 1992. Formation of a bispecific antibody by the use of leucine zippers. J. Immunol. 148: 1547–1553.
Thomas, G.D., Chappell, M.J., Dykes, P.W., Ramsden, D.B., Godfrey, K.R., Ellis, J.R.M. and Bradwell, A.R. 1989. Effect of dose, molecular size, affinity, and protein uptake of antibody or ligand: a biomathematical model. Cancer Res. 49: 3290–3296.
Huston, J.S., McCartney, J.S., Tai, M.-S., Mottola-Hartshorn, C., Jin, D., Warren, F., Keck, P. and Opperman, H. 1993. Medical applications of single-chain antibodies. Intern. Rev. of Immunol. In press
Maurer, R., Meyer, B.J. and Ptashne, M. 1980. Gene regulation at the right operator (O R) of bacteriophage λ-O R3 and autogenous negative control by represser. J. Mol. Biol. 139: 147–161.
Carter, P., Kelley, R.F., Rodrigues, M.L., Snedecor, B., Covarrubias, M., Velligan, M.D., Wong, W.L.T., Rowland, A.M., Kotts, C.E., Carver, M.E., Yang, M., Bourell, J.H., Shepard, H.M. and Henner, D. 1992. High level Escherichia coli expression and production of a bivalent humanized antibody fragment. Bio/Technology 10: 163–167.
Sambrook, J., Fritsch, E.F. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
Hopp, T.P., Prickett, K.S., Price, V.L., Libby, R.T., March, C.J., Cerretti, C.P., Urdal, D.L. and Conlon, P.J. 1988. A short polypeptide marker sequence useful for recombinant protein identification and purification. Bio/Technology 6: 1204–1210.
Riesenberg, D., Menzel, K., Schulz, V., Schumann, K., Veith, G., Zuber, G. and Knorre, W.A. 1990. High cell density fermentation of recombinant Escherichia coli expressing human interferon alpha 1. Appl. Microbiol. Biotechnol. 34: 77–82.
Löser, C. 1990. Stabilitätsverhalten eines rekombinanten ColEl-Plasmid-derivates mit Ampicillin-Resistenzmarker und Human-Interferon-α-1-Gen in Escherichia coli . PhD-Thesis. Technical University Dresden, Fed. Rep. of Germany.
Bignon, C. and Martin, P.-M. 1992. Improved sequencing of “mini-prep” double-stranded templates using a multiwell microplate system. Biotechniques 13: 104–106.
Neidhardt, F.C. 1987. Chemical composition of Escherichia coli. 3–6. In: Escherichia and Salmonella typhimurium. Cellular and Molecular Biology. F. C. Neidhardt (Ed. in chief). ASM Washington, D.C.
Lowry, O.H., Rosebrough, N.J., Fair, A.L. and Randall, R.J. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193: 265–275.
Layne, E. 1957. Spectrophotometric and turbidimetric methods for measuring proteins. Meth. Enzymol. 3: 447–454.
Gill, S.T. and von Hippel, P.H. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182: 319–326.
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685.
Burton, D.R. 1985. Immunoglobulin G: functional sites. Mol. Immunol. 22: 161–182.
Dangl, J.L., Wensel, T.G., Morrison, S.L., Stryer, L., Herzenberg, L.A. and Oi, V.T. 1988. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 7: 1989–1994.
Lupas, A., Van Dyke, M. and Stock, J. 1991. Predicting coiled coils from protein sequences. Science 252: 1162–1164.
Freund, C., Ross, A., Guth, B., Plückthun, A. and Holak, T. 1993. Characterization of the linker peptide of the single-chain Fv fragment of an antibody by NMR spectroscopy. FEBS Lett. 320: 97–100.
Banner, D.W., Kokkinidis, M. and Tsernoglou, D. 1987. Structure of the ColE1 Rop protein at 1.7Å resolution. J. Mol. Biol. 196: 657–675.
Kraulis, P.J. 1991. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Cryst. 24: 946–950.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pack, P., Kujau, M., Schroeckh, V. et al. Improved Bivalent Miniantibodies, with Identical Avidity as Whole Antibodies, Produced by High Cell Density Fermentation of Escherichia coli. Nat Biotechnol 11, 1271–1277 (1993). https://doi.org/10.1038/nbt1193-1271
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt1193-1271
This article is cited by
-
High-level production of a single chain antibody against anthrax toxin in Escherichia coli by high cell density cultivation
Bioprocess and Biosystems Engineering (2011)
-
Generation of Rabbit Monoclonal Antibody Fragments from a Combinatorial Phage Display Library and Their Production in the Yeast Pichia pastoris
Nature Biotechnology (1995)