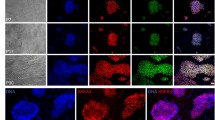
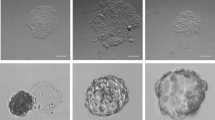
Abstract
We have previously reported that high concentrations of basic fibroblast growth factor (bFGF) support feeder-independent growth of human embryonic stem (ES) cells, but those conditions included poorly defined serum and matrix components. Here we report feeder-independent human ES cell culture that includes protein components solely derived from recombinant sources or purified from human material. We describe the derivation of two new human ES cell lines in these defined culture conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Puck, T.T. & Marcus, P.I. Proc. Natl. Acad. Sci. USA 4, 432–437 (1955).
Thomson, J.A. et al. Science 282, 1145–1147 (1998).
Ying, Q.L., Nichols, J., Chambers, I. & Smith, A. Cell 115, 281–292 (2003).
Xu, R.H. et al. Nat. Biotechnol. 20, 1261–1264 (2002).
Xu, R.H. et al. Nat. Methods 2, 185–190 (2005).
Amit, M. et al. Dev. Biol. 227, 271–278 (2000).
Klimanskaya, I. et al. Lancet 365, 1636–1641 (2005).
Xu, C. et al. Stem Cells 23, 315–323 (2005).
Martin, M.J., Muotri, A., Gage, F. & Varki, A. Nat. Med. 11, 228–232 (2005).
Sperger, J.M. et al. Proc. Natl. Acad. Sci. USA 100, 13350–13355 (2003).
Amit, M., Shariki, C., Margulets, V. & Itskovitz-Eldor, J. Biol. Reprod. 70, 837–845 (2004).
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P. & Brivanlou, A.H. Nat. Med. 10, 55–63 (2004).
Watanabe, M., Maemura, K., Kanbara, K., Tamayama, T. & Hayasaki, H. Int. Rev. Cytol. 213, 1–47 (2002).
Takahama, K. et al. Neuropharmacology 25, 339–342 (1986).
Draper, J.S. et al. Nat. Biotechnol. 22, 53–54 (2004).
Acknowledgements
NIH grants R24-RR017721 and P20-GMO69981 only supported work with federally approved cell lines H1, H7, H9 and H14. New human ES cell derivations and analysis was performed exclusively at WiCell, using privately funded WiCell facilities, equipment and personnel. We thank S. Lindheim for consenting patients who donated their embryos to this research, and D. Faupel for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.A.T. is cofounder and owns stock in Cellular Dynamics International. The remaining authors declare that they have no competing financial interests.
Supplementary information
Supplementary Fig. 1
Optimization of physiochemical culture conditions for human ES cells (PDF 1158 kb)
Supplementary Fig. 2
Karyotype analysis of human ES cells cultured in TeSR1 medium (PDF 421 kb)
Supplementary Fig. 3
Pluripotency of human ES cells maintained in TeSR1 medium (PDF 518 kb)
Supplementary Fig. 4
Representative morphology and FACS analysis of human ES cells cultured in defined, feeder-independent conditions. (PDF 604 kb)
Supplementary Fig. 5
Absence of sialic acid contamination in human ES cells cultured in defined conditions (PDF 102 kb)
Supplementary Fig. 6
Karyotype of new human ES cell lines (PDF 212 kb)
Supplementary Table 1
Complete formulation for TeSR1 medium (PDF 30 kb)
Rights and permissions
About this article
Cite this article
Ludwig, T., Levenstein, M., Jones, J. et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol 24, 185–187 (2006). https://doi.org/10.1038/nbt1177
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1177
This article is cited by
-
Learning Towards Maturation of Defined Feeder-free Pluripotency Culture Systems: Lessons from Conventional Feeder-based Systems
Stem Cell Reviews and Reports (2024)
-
SARS-CoV-2 viral genes Nsp6, Nsp8, and M compromise cellular ATP levels to impair survival and function of human pluripotent stem cell-derived cardiomyocytes
Stem Cell Research & Therapy (2023)
-
Efficient and safe single-cell cloning of human pluripotent stem cells using the CEPT cocktail
Nature Protocols (2023)
-
Temporal morphogen gradient-driven neural induction shapes single expanded neuroepithelium brain organoids with enhanced cortical identity
Nature Communications (2023)
-
Development and evaluation of a novel xeno-free culture medium for human-induced pluripotent stem cells
Stem Cell Research & Therapy (2022)