Abstract
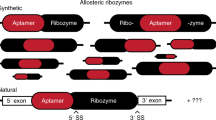
Allosteric RNAs operate as molecular switches that alter folding and function in response to ligand binding. A common type of natural allosteric RNAs is the riboswitch; designer RNAs with similar properties can be created by RNA engineering. We describe a computational approach for designing allosteric ribozymes triggered by binding oligonucleotides. Four universal types of RNA switches possessing AND, OR, YES and NOT Boolean logic functions were created in modular form, which allows ligand specificity to be changed without altering the catalytic core of the ribozyme. All computationally designed allosteric ribozymes were synthesized and experimentally tested in vitro. Engineered ribozymes exhibit >1,000-fold activation, demonstrate precise ligand specificity and function in molecular circuits in which the self-cleavage product of one RNA triggers the action of a second. This engineering approach provides a rapid and inexpensive way to create allosteric RNAs for constructing complex molecular circuits, nucleic acid detection systems and gene control elements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Breaker, R.R. Natural and engineered oligonucleotides as tools to explore biology. Nature 432, 838–845 (2004).
Gold, L., Polisky, B., Uhlenbeck, O.C. & Yarus, M. Diversity of oligonucleotides functions. Annu. Rev. Biochem. 64, 763–797 (1995).
Osborne, S.E. & Ellington, A.D. Nucleic acid selection and the challenge of combinatorial chemistry. Chem. Rev. 97, 349–370 (1997).
Hamaguchi, N., Ellington, A. & Stanton, M. Aptamer beacons for the detection of proteins. Anal. Biochem. 294, 126–131 (2001).
McCauley, T.G., Hamaguchi, N. & Stanton, M. Aptamer-based biosensor arrays for detection and quantification of biological macromolecules. Anal. Biochem. 319, 244–250 (2003).
Bock, C. et al. Photoaptamer arrays applied to multiplexed proteomic analysis. Proteomics 4, 609–618 (2004).
Soukup, G.A. & Breaker, R.R. Nucleic acid molecular switches. Trends Biotechnol. 17, 469–476 (1999).
Soukup, G.A. & Breaker, R.R. Allosteric nucleic acid catalysts. Curr. Opin. Struct. Biol. 10, 318–325 (2000).
Silverman, S.K. Rube Goldberg goes (ribo)nuclear? Molecular switches and sensors made from RNA. RNA 9, 377–383 (2003).
Seetharaman, S., Zivarts, M., Sudarsan, N. & Breaker, R.R. Immobilized RNA switches for the analysis of complex chemical and biological mixtures. Nat. Biotechnol. 19, 336–341 (2001).
Hesselberth, J.R., Robertson, M.P., Knudsen, S.M. & Ellington, A.D. Simultaneous detection of diverse analytes with an aptazyme ligase array. Anal. Biochem. 312, 106–112 (2003).
Nahvi, A. et al. Genetic control by a metabolite binding mRNA. Chem. Biol. 9, 1043–1049 (2002).
Winkler, W., Nahvi, A. & Breaker, R.R. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 419, 952–956 (2002).
Mironov, A.S. et al. Sensing small molecules by nascent RNA: a mechanism to control transcription in bacteria. Cell 111, 747–756 (2002).
Winkler, W.C., Cohen-Chalamish, S. & Breaker, R.R. An mRNA structure that controls gene expression by binding FMN. Proc. Natl. Acad. Sci. USA 99, 15908–15913 (2002).
Sudarsan, N., Barrick, J.E. & Breaker, R.R. Metabolite-binding RNA domains are present in the genes of eukaryotes. RNA 9, 644–647 (2003).
Kubodera, T. et al. Thiamine-regulated gene expression of Aspergillus oryzae thiA requires splicing of the intron containing a riboswitch-like domain in the 5′ UTR. FEBS Lett. 555, 516–520 (2003).
Winkler, W.C. & Breaker, R.R. Genetic control by metabolite-binding riboswitches. ChemBioChem 4, 1024–1032 (2003).
Vitreschak, A.G., Rodionov, D.A., Mironov, A.A. & Gelfand, M.S. Riboswitches: the oldest mechanism for the regulation of gene expression. Trends Genet. 20, 44–50 (2004).
Mandal, M. & Breaker, R.R. Gene regulation by riboswitches. Nat. Rev. Mol. Cell Biol. 5, 451–463 (2004).
Lewin, A.S. & Hauswirth, W.W. Ribozyme gene therapy: applications for molecular medicine. Trends Mol. Med. 7, 221–228 (2001).
Schubert, S. & Kurreck, J. Ribozyme- and deoxyribozymes-strategies for medical applications. Curr. Drug Targets 5, 667–681 (2004).
Werstuck, G. & Green, M.R. Controlling gene expression in living cells through small molecule-RNA interactions. Science 282, 296–298 (1998).
Grate, D. & Wilson, C. Inducible regulation of the S. cerevisiae cell cycle mediated by an RNA aptamer-ligand complex. Bioorg. Med. Chem. 9, 2565–2570 (2001).
Thompson, K.M., Syrett, H.A., Knudsen, S.M. & Ellington, A.D. Group I aptazymes as genetic regulatory switches. BCM Biotechnol. 2, 21 (2002).
Suess, B., Fink, B., Berens, C., Stenz, R. & Hillen, W. A theophylline responsive riboswitch based on helix slipping controls gene expression in vivo. Nucleic Acids Res. 32, 1610–1614 (2004).
Desai, S.K. & Gallivan, J.P. Genetic screens and selections for small molecules based on a synthetic riboswitch that activates protein translation. J. Am. Chem. Soc. 126, 13247–13254 (2004).
Breaker, R.R. Engineered allosteric ribozymes as biosensor components. Curr. Opin. Biotechnol. 13, 31–39 (2002).
Ferguson, A. et al. A novel strategy for selection of allosteric ribozymes yields RiboReporter sensors for caffeine and aspartame. Nucleic Acids Res. 32, 1756–1766 (2004).
Srinivasan, J. et al. ADP-specific sensors enable universal assay of protein kinase activity. Chem. Biol. 11, 499–508 (2004).
Stojanovic, M.N. & Stefanovic, D. Deoxyribozyme-based half-adder. J. Am. Chem. Soc. 125, 6673–6676 (2003).
Stojanovic, M. & Stafanovic, D. A deoxyribozyme-based molecular automaton. Nat. Biotechnol. 21, 1069–1074 (2003).
Tang, J. & Breaker, R.R. Rational design of allosteric ribozymes. Chem. Biol. 4, 453–459 (1997).
Jose, A.M., Soukup, G.A. & Breaker, R.R. Cooperative binding of effectors by an allosteric ribozyme. Nucleic Acids Res. 29, 1631–1637 (2001).
Soukup, G.A. & Breaker, R.R. Engineering precision RNA molecular switches. Proc. Natl. Acad. Sci. USA 96, 3584–3589 (1999).
Koizumi, M., Kerr, J.K., Soukup, G.A. & Breaker, R.R. Allosteric ribozymes sensitive to the second messengers cAMP and cGMP. Nat. Struct. Biol. 6, 1062–1071 (1999).
Robertson, M.P., Knudsen, S.M. & Ellington, A.D. In vitro selection of ribozymes dependent on peptides for activity. RNA 10, 114–127 (2004).
Porta, H. & Lizardi, P.M. An allosteric hammerhead ribozyme. Biotechnol. 13, 161–164 (1995).
Komatsu, Y., Yamashita, S., Kazama, N., Nobuoka, K. & Ohtsuka, E. Constructuion of new ribozymes requiring short regulator oligonucleotides as a cofactor. J. Mol. Biol. 299, 1231–1243 (2000).
Burke, D.H., Ozerova, N.D. & Nilsen-Hamilton, M. Allosteric hammerhead ribozyme TRAPs. Biochemistry 41, 6588–6594 (2002).
Wickiser, J.K., Cohen-Chalamish, S., Puskarz, I., Sawiki, B., Ward, D.C. & Breaker, R.R. Engineered oligonucleotides-responsive ribozymes that form structures resembling kissing complexes. (in preparation).
Stojanovic, M.N., Mitchell, T.E. & Stefanovic, D. Deoxyribozyme-based logic gates. J. Am. Chem. Soc. 124, 3555–3561 (2002).
Wang, D.Y., Lai, B.H., Feldman, A.R. & Sen, D. A general approach for the use of oligonucleotides effectors to regulate the catalysis of RNA-cleaving ribozymes and DNAzymes. Nucleic Acids Res. 30, 1735–1742 (2002).
McCaskill, J.S. The equilibrium partition function and base pair binding probabilities for RNA secondary structure. Biopolymers 29, 1109–1119 (1990).
Bonhoeffer, S., McCaskill, J.S., Stadler, P.F. & Schuster, P. RNA multi-structure landscapes. A study based on temperature dependent partition functions. Eur. Biophys. J. 22, 13–24 (1993).
Russell, R. et al. Exploring the folding landscape of a structured RNA. Proc. Natl. Acad. Sci. USA 99, 155–160 (2002).
Woodson, S.A. Folding mechanisms of group I ribozymes: Role of stability and contact order. Biochem. Soc. Trans. 30, 1166–1169 (2002).
Sosnick, T.R. & Pan, T. RNA folding: Models and perspectives. Curr. Opin. Struct. Biol. 13, 309–316 (2003).
Flamm, C., Fontana, W., Hofacker, I. & Schuster, P. RNA folding kinetics at elementary step resolution. RNA 6, 325–338 (2000).
Flamm, C., Hofacker, I.L., Maurer-Stroh, Stadler, P.F. & Zehl, M.S. Design of multi-stable RNA molecules. RNA 7, 254–265 (2001).
Forster, A.C. & Symons, R.H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell 49, 211–220.
Hertel, K.J. et al. Numbering system for the hammerhead. Nucleic Acids Res. 20, 3252 (1992).
Osborne, E.M., Schaak, J.E. & DeRose, V.J. Characterization of a native hammerhead ribozyme derived from schistosomes. RNA 11, 187–196 (2005).
Khvorova, A., Lescoute, A., Westhof, E. & Jayasena, S.D. Sequence elements outside the hammerhead ribozyme catalytic core enable intracellular activity. Nat. Struct. Biol. 10, 708–712 (2003).
De la Pena, M., Gago, S. & Flores, R. Peripheral regions of natural hammerhead ribozymes greatly increase their self-cleavage activity. EMBO J. 22, 5561–5570 (2003).
Canny, M.D. et al. Fast cleavage kinetics of a natural hammerhead ribozyme. J. Am. Chem. Soc. 126, 10848–10849 (2004).
Mandal, M. et al. A glycine-dependent riboswitch that uses cooperative binding to control gene expression. Science 306, 275–279 (2004).
Yen, L. et al. Exogenous control of mammalian gene expression through modulation of RNA self-cleavage. Nature 431, 471–476 (2004).
Bayer, T.S. & Smolke, C.D. Programmable ligand-controlled riboregulators of eukaryotic gene expression. Nat. Biotechnol. 23, 337–343 (2005).
Isaacs, F.J. et al. Engineered riboregulators enable post-transcriptional control of gene expression. Nat. Biotechnol. 22, 841–847 (2004).
Penchovsky, R. & Ackermann, J. DNA library design for molecular computation. J. Comput. Biol. 10, 215–229 (2003).
Mathews, D., Sabina, J., Zucker, M. & Turner, H. Expanded sequence dependence of thermodynamic parameters provides robust prediction of RNA secondary structure. J. Mol. Biol. 288, 911–940 (1999).
Hofacker, I.L. et al. Fast folding and comparison of RNA secondary structures. Monatsh. Chem. 125, 167–188 (1994).
Hofacker, I.L. Vienna RNA secondary structure server. Nucleic Acids Res. 31, 3429–3431 (2003).
Acknowledgements
We thank members of the Breaker laboratory for helpful discussions. This work was supported by grants from the National Science Foundation and by the Defense Advance Research Projects Agency (DARPA). This project also was supported in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, under contract No. N01-HV-28186.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
R.R.B. is cofounder of the biotechnology company Archemix (Cambridge, MA, USA) that holds intellectual property on RNA switch technology.
Supplementary information
Supplementary Fig. 1
Design and characterization of YES-3, a variant of YES-1 that exhibits altered oligonucleotide specificity. (PDF 403 kb)
Supplementary Fig. 2
Design and characterization of YES-4, a variant of YES-1 that has altered oligonucleotide specificity. (PDF 398 kb)
Supplementary Fig. 3
Design and characterization of YES-5, a variant of YES-1 that exhibits altered oligonucleotide specificity. (PDF 404 kb)
Supplementary Fig. 4
Design and characterization of YES-6, which is more thermodynamically stable (Ep = −57 kcal mol−1) than the other YES switches. It is design not to cleave even in the presence of corresponding DNA effector. (PDF 160 kb)
Supplementary Fig. 5
Dot plot matrix for NOT-1. See Fig. 3 for details on the sequence and function of NOT-1. (PDF 174 kb)
Supplementary Fig. 6
Dot plot matrix for AND-1. (PDF 282 kb)
Supplementary Fig. 7
Dot plot matrix for OR-1. (PDF 418 kb)
Supplementary Fig. 8
Design and function of three variants of OR-1 that exhibit improved levels of oligonucleotide-triggered self cleavage. (PDF 526 kb)
Supplementary Fig. 9
A flow chart of the computational procedure for the design of RNA switches. (PDF 171 kb)
Rights and permissions
About this article
Cite this article
Penchovsky, R., Breaker, R. Computational design and experimental validation of oligonucleotide-sensing allosteric ribozymes. Nat Biotechnol 23, 1424–1433 (2005). https://doi.org/10.1038/nbt1155
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1155
This article is cited by
-
Evolutionary design and analysis of ribozyme-based logic gates
Genetic Programming and Evolvable Machines (2023)
-
Synthetic DNA applications in information technology
Nature Communications (2022)
-
An analysis of simple computational strategies to facilitate the design of functional molecular information processors
BMC Bioinformatics (2016)
-
Multi-objective optimization for RNA design with multiple target secondary structures
BMC Bioinformatics (2015)
-
In-silico design of computational nucleic acids for molecular information processing
Journal of Cheminformatics (2013)