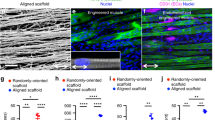
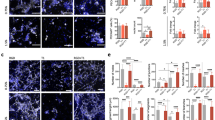
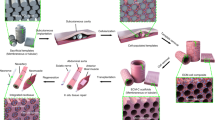
Abstract
One of the major obstacles in engineering thick, complex tissues such as muscle is the need to vascularize the tissue in vitro. Vascularization in vitro could maintain cell viability during tissue growth, induce structural organization and promote vascularization upon implantation. Here we describe the induction of endothelial vessel networks in engineered skeletal muscle tissue constructs using a three-dimensional multiculture system consisting of myoblasts, embryonic fibroblasts and endothelial cells coseeded on highly porous, biodegradable polymer scaffolds. Analysis of the conditions for induction and stabilization of the vessels in vitro showed that addition of embryonic fibroblasts increased the levels of vascular endothelial growth factor expression in the construct and promoted formation and stabilization of the endothelial vessels. We studied the survival and vascularization of the engineered muscle implants in vivo in three different models. Prevascularization improved the vascularization, blood perfusion and survival of the muscle tissue constructs after transplantation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Saxena, A.K., Marler, J., Benvenuto, M., Willital, G.H. & Vacanti, J.P. Skeletal muscle tissue engineering using isolated myoblasts on synthetic biodegradable polymers: preliminary studies. Tissue Eng. 5, 525–532 (1999).
Neumann, T., Nicholson, B.S. & Sanders, J.E. Tissue engineering of perfused microvessels. Microvasc. Res. 66, 59–67 (2003).
Bach, A.D., Stem-Straeter, J., Beier, J.P., Bannasch, H. & Stark, G.B. Engineering of muscle tissue. Clin. Plast. Surg. 30, 589–599 (2003).
Wigmore, P.M. & Evans, D.J. Molecular and cellular mechanisms involved in the generation of fiber diversity during myogenesis. Int. Rev. Cytol. 216, 175–232 (2002).
Buckingham, M. Skeletal muscle formation in vertebrates. Curr. Opin. Genet. Dev. 11, 440–448 (2001).
Zisch, A.H., Lutolf, M.P. & Hubbell, J.A. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc. Pathol. 12, 295–310 (2003).
von Degenfeld, G., Banfi, A., Springer, M.L. & Blau, H.M. Myoblast-mediated gene transfer for therapeutic angiogenesis and arteriogenesis. Br. J. Pharmacol. 140, 620–626 (2003).
Lu, Y. et al. Recombinant vascular endothelial growth factor secreted from tissue-engineered bioartificial muscles promotes localized angiogenesis. Circulation 104, 594–599 (2001).
Levenberg, S., Golub, J.S., Amit, M., Itskovitz-Eldor, J. & Langer, R. Endothelial cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 99, 4391–4396 (2002).
Levenberg, S. et al. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc. Natl. Acad. Sci. USA 100, 12741–12746 (2003).
Darland, D.C. & D'Amore, P.A. TGF beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis 4, 11–20 (2001).
Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 9, 653–660 (2003).
Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 (2003).
Sieminski, A.L., Padera, R.F., Blunk, T. & Gooch, K.J. Systemic Delivery of Human Growth Hormone Using Genetically Modified Tissue-Engineered Microvascular Networks: Prolonged Delivery and Endothelial Survival with Inclusion of Nonendothelial Cells. Tissue Eng. 8, 1057–1069 (2002).
Koike, N. et al. Tissue engineering: creation of long-lasting blood vessels. Nature 428, 138–139 (2004).
Black, A.F., Berthod, F., L'Heureux, N., Germain, L. & Auger, F.A. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 12, 1331–1340 (1998).
Shinoka, T. Tissue engineered heart valves: autologous cell seeding on biodegradable polymer scaffold. Artif. Organs 26, 402–406 (2002).
Flamme, I., Frolich, T. & Risau, W. Molecular mechanisms of vasculogenesis and embryonic angiogenesis. J. Cell. Physiol. 173, 206–210 (1997).
Rossant, J. & Hirashima, M. Vascular development and patterning: making the right choices. Curr. Opin. Genet. Dev. 13, 408–412 (2003).
Hirschi, K.K., Rohovsky, S.A. & D'Amore, P.A. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J. Cell Biol. 141, 805–814 (1998).
Darland, D.C. et al. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev. Biol. 264, 275–288 (2003).
Lammert, E., Cleaver, O. & Melton, D. Induction of pancreatic differentiation by signals from blood vessels. Science 294, 564–567 (2001).
Matsumoto, K., Yoshitomi, H., Rossant, J. & Zaret, K.S. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 294, 559–563 (2001).
Yaffe, D. & Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270, 725–727 (1977).
Blau, H.M. et al. Plasticity of the differentiated state. Science 230, 758–766 (1985).
Holder, W.D. Jr. et al. Cellular ingrowth and thickness changes in poly-L-lactide and polyglycolide matrices implanted subcutaneously in the rat. J. Biomed. Mater. Res. 41, 412–421 (1998).
Acknowledgements
The authors thank the MIT division of comparative medicine for excellent assistance in tissue embedding and processing, Adam Kapur for help with data analysis, and Justin S. Golub for help with RT-PCR assays. We would like to thank Joseph Itskovitz-Eldor for assistance and cooperation in conducting this research. This work was supported by National Institutes of Health grants HL60435 (R.L. and S.L.) and EY05318 (P.A.D. and D.C.D.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
VEGF and PDGF-B expression in 3D constructs. (PDF 873 kb)
Supplementary Fig. 2
Quantitative analysis of number of endothelial vessels in muscle implants seeded with HUVEC or hESC-derived endothelial cells. (PDF 481 kb)
Rights and permissions
About this article
Cite this article
Levenberg, S., Rouwkema, J., Macdonald, M. et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol 23, 879–884 (2005). https://doi.org/10.1038/nbt1109
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1109
This article is cited by
-
Motor neurons and endothelial cells additively promote development and fusion of human iPSC-derived skeletal myocytes
Skeletal Muscle (2024)
-
The interplay of cells, polymers, and vascularization in three-dimensional lung models and their applications in COVID-19 research and therapy
Stem Cell Research & Therapy (2023)
-
Engineered tissue vascularization and engraftment depends on host model
Scientific Reports (2023)
-
Co-culture approaches for cultivated meat production
Nature Reviews Bioengineering (2023)
-
Engineering the multiscale complexity of vascular networks
Nature Reviews Materials (2022)