Abstract
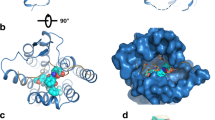
We report the generation of superactive analogues of human glycoprotein hormones, with potential applications in thyroid and reproductive disorders. Current biological and structural data were used to rationalize mutagenesis. The 11–20 region in the α-subunit with a cluster of lysine residues forms a previously unrecognized domain critical for receptor binding and signal transduction, as well as an important motif in the evolution of glycoprotein hormone activities. The gradual elimination of basic residues in the α-subunit coincided with the evolutionary divergence of the hominids from the Old World monkeys. By selective reconstitution of certain critical residues present in homologous nonhuman hormones we have developed human thyroid stimulating hormone and chorionic gonadotropin analogues with substantial increases in receptor binding affinity and bioactivity, thus providing a paradigm for the design of novel therapeutic protein analogues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Pierce, J.G. and Parsons, T.F. 1981. Glycoprotein hormones: structure and function. Ann. Rev. Biochem. 50: 465–495.
Fontaine, Y-A. and Burzawa-Gerard, E. 1977. Esquisse de l'evolution des hormones gonadotopes et thyreotropes des vertebres. Gen. Comp. Endocrinol. 32: 341–347.
Litch, P., Papkoff, H., Farmer, S.W., Muller, Ch.H., Tsui, H.W., and Crews, D. 1977. Evolution of gonadotropin structure and function. Rec. Progr. Horm. Res. 33: 169–248.
Combarnous, Y. . 1992. Molecular basis of the specificity of binding of glycoprotein hormones to their receptors. Endocrine Rev. 13: 670–691.
Yamazaki, K., Sato, K., Shizume, K., Ito, Y., Obara, T., et al. 1995. Potent thyrotropic activity of human chorionic gonadotropin variants in terms of 125I incorporation and de novo synthesized thyroid hormone release in human thyroid follicles. J. Clin. Endocrinol. Metab. 80: 473–479.
Fares, F.A., Suganuma, N., Nishimori, K., LaPolt, P.S., Hsueh, A.J.W. and Boime, I. 1992 Design of a long-acting follitropin agonist by fusing the C-terminal sequence of the chorionic gonadotropin β subunit to the follitropin β subunit. Proc. Natl. Acad Sci. USA 89: 4304–4308.
LaPolt, P.S., Nishimori, K., Fares, F.A., Perlas, E., Boime, I., and Hsueh, A.J.W. 1992. Enhanced stimulation of follicle maturation and ovulatory potential by long acting follicle-stimulating hormone agonists with extended carboxyl-terminal peptides. Endocrinology 131: 2514–2520.
Sugahara, T., Pixley, M.R., Minami, S., Perlas, E., Ben-Menahem, D., Hsueh, A.J.W. et al. 1995. Biosynthesis of a biologically active single peptide chain containing the common α and chorionic gonadotropin β subunits in tandem. Proc. Natl. Acad Sci. USA 92: 2041–2045.
Joshi, L., Murata, Y., Wondisford, F.E., Szkudlinski, M.W., Desai, R., and Weintraub, B.D. 1995. Recombinant thyrotropin containing a β-subunit chimera with the human chorionic gonadotropin-β carboxy terminus is biologically active, with a prolonged plasma half-life: role of carbohydrate in bioactivity and metabolic clearance. Endocrinology 136: 3839–3848.
Szkudlinski, M.W., Thotakura, N.R. and Weintraub, B.D. 1995. Subunit-specific functions of N-linked oligosaccharides in human thyrotropin: role of terminal residues of α-subunit and β-subunit oligosaccharides in metabolic clearance and bioactivity. Proc. Natl. Acad. Sci. USA 92: 9062–9066.
Lapthorn, A.J., Harris, D.C., Littlejohn, A., Lustbader, J.W., Canfield, R.E., Machin, K.J., Morgan, F.J. and Isaacs, N.W. 1994. Crystal structure of human chorionic gonadotropin. Nature 369: 455–461.
Wu, H., Lustbader, J.W., Liu, Y., Canfield, R.E., and Hendrickson, W.A 1994. Structure of human chorionic gonadotropin at 2.6 A resolution from MAD analysis of the selenomethionyl protein. Structure 2: 545–558.
Strader, C.D., Sigal, I.S. and Dixon, R.A.F. 1989. Structural basis of β-adrenergic receptor function. FASEB J. 3: 1825–1832.
Ostrowski, J., Kjelsberg, M.A., Caron, M.G. and Lefkowitz, R.J. 1992. Mutagenesis of the β2-adrenergic receptor: how structure elucidates function. Annu. Rev. Pharmacol. Toxicol. 32: 167–183.
Ji, I., Zeng H., and Ji, T.H. 1993. Receptor activation of and sgnal generation by the lutropin/choriogonadotropin receptor. J. Biol. Chem. 268: 22971–22974.
Rao, V.R., Cohen, G.B., and Oprian, D.D. 1994. Rhodopsin mutation G90D and a molecular mechanism for congenital blindness. Nature 367: 639–641.
Golos, T.G., Durning, M. and Fisher, J.M. 1991. Molecular cloning of the Rhesus glycoprotein hormone α-subunit gene. DNA Cell. Biol. 10: 367–380.
Stanton, P.G. and Hearn, M.T.W. 1987. The iodination sites of bovine thyrotropin. J. Biol. Chem. 262: 1623–1632.
Dimhofer, S., Lechner, O., Madersbacher, S., Kieber, R., de Leeuw, R., Wick, G., et al. 1994. Free α-subunit of human chorionic gonadotrophin: molecular basis of immunologically and biologically active domains. J. Endocrine*. 140: 145–154.
Liu, W.K., Yang, K.P., Nakagawa Y., and Ward, D.N. 1974. The role of the amino group in the subunit association and receptor site interaction for ovine luteinizing hormone as studied by acylation. J. Biol. Chem. 249: 5544–5550.
Yadav, S.P., Brew, K., Majercik, M.H. and Puett, D. 1994. Holoprotein formation of human chorionic gonadotropin: differential trace labeling with acetic anhydride. Mol. Endocrinol. 8: 1547–1558.
Grossmann, M., Szkudlinski, M.W., Zeng, H., Kraiem, Z., Ji, I., Tropea, J.E. et al. 1995. Role of the carboxy-terminal residues of the α-subunit in the expression and bioactivity of human thyroid-stimulating hormone. Mol. Endocrinol. 9: 948–958.
Moyle, W.R., Campbell, R.K., Venkateswara Rao, S.N., Ayad, N.G., Bernard, M.R., Han, Y., et al. 1995. Model of human chorionic gonadotropin and lutropin receptor interaction that explains signal transduction of the glycoprotein hormones. J. Biol. Chem. 270: 20020–20031.
Jiang, X., Dreano, M., Buckler, D.R., Cheng, S., Ythier, A., Wu, H., et al. 1995. Structural predictions for the ligand-binding region of glycoprotein hormone receptors and the nature of hormone-receptor interactions. Structure 3: 1341–1353.
Kajava, A.V., Vassart, G. and Wodak, S.J. 1995. Modeling of the three-dimensional structure of proteins with the typical leucine-rich repeats. Structure 3: 867–877.
Ryden, M., Murray-Rust, J., Glass, D., LLag L.L., Trupp, M., Yancopoulos, G.D., et al. 1995. Functional analysis of mutant neurotrophins deficient in low affinity binding reveals a role for p75LNGFR in NT-4 signalling. EMBO J. 14: 1979–1990.
Griffith, D.L., Keck, P.C., Sampath, T., Rueger, D.C., and Carlson, W.D. 1996. Three-dimensional structure of recombinant human osteogenic protein 1: structural paradigm for the transforming growth factor 13 superfamily. Proc. Natl. Acad. Sci. USA 93: 878–883.
Meier, C.A., Braverman, L.E., Ebner, S.A., Veronikis, I., Daniels, G.H., Ross, D.S., et al. 1994. Diagnostic use of recombinant human thyrotropin in patients with thyroid car-cinoma (Phase l/ll study). J. Clin. Endocrinol. Metab. 78: 188–196.
Gast, M.J. 1995. Evolution of clinical agents for ovulation induction. Am. J. Obstet. Gynecol. 172 (suppl., part 2): 753–759.
Whitcomb, R.W. and Crowley, W.F. 1990. Diagnosis and treatment of isolated gonadotropin-releasing hormone deficiency in men. J. Clin. Endocrinol. Metab. 70: 3–7.
Ben-Rafael, Z., Levy, T., and Schoemaker, J. 1995. Pharmacokinetics of follicle-stimulating hormone: clinical significance. Fertil. Steril. 63: 689–700.
Lunardi-Iskandar, Y., Bryant, J.L., Zeman, R.A., Lam, V.H., Samaniego, R., Besnier, J.M., et al. 1995. Tumorigenesis and metastasis of neoplastic Kaposi's sarcoma cell line in immunodeficient mice blocked by a human pregnancy hormone. Nature 375: 64–68.
Moyle, W.R., Campbell, R.K., Myers, R.V., Bernard, M.R., Han, Y., and Wang, X. 1994. Co-evolution of ligand-receptor pairs. Nature 268: 251–255.
Tullner, W.W. 1994. Comparative aspects of primate chorionic gonadotropins. Contrib. Primat. 3: 235–257.
Condliffe, P.G. and Weintraub, B.D. 1979. Pituitary thyroid-stimulating hormone and other thyroid stimulating substances, pp. 499–574 in Hormones in blood, 3rd ed., Gray, C.H. and James, V.H.T. (eds.). Academic Press.
East-Palmer, J., Szkudlinski, M.W., Lee, J., Thotakura, N.R. and Weintraub, B.D. 1995. A novel, nonradioactive in vivo bioassay of thyrotropin (TSH). Thyroid 5: 55–59.
Liu, C., Roth, K.E., Lindau-Shepard, B.A., Shaffer, J.B. and Dias, J.A. 1994. Site-directed alanine mutagenesis of Phe33, Arg35, and Arg42-Ser43-Lys44 in the human gonadotropin α-subunit. J. Biol. Chem. 269: 25289–25294.
Szkudlinski, M.W., Grossmann, M. and Weintraub, B.D. 1996. Structure-function studies of human TSH: new advances in design of glycoprotein hormone analogs. Trends Endocrinol. Metab. 7: 277–286.
Campbell, R.K., Dean-Emig, D.M. and Moyle, W.R. 1991. Conversion of human choriog-onadotropin into a follitropin by protein engineering. Proc. Natl. Acad Sci. USA 88: 760–764.
Dias, J.A., Zhang, Y., and Liu, X. 1994. Receptor binding and functional properties of chimeric human follitropin prepared by an exchange between a small hydrophilic intercysteine loop of human follitropin and human lutropin. J. Biol. Chem. 269: 25289–25294.
Fiddes, J.C. and Goodman, H.M. 1979. Isolation, cloning and sequence analysis of the cDNA for the α-subunit of human chorionic gonadotropin. Nature 281: 351–356.
Sarkar, G. and Sommer, S.S. 1990. The “megaprimer” method of site-directed mutagenesis. BioTechniques 8: 404–407.
Szkudlinski, M.W., Thotakura, N.R., Bucci, I., Joshi, L.R., Tsai, A., East-Palmer, J., et al. 1993. Purification and characterization of recombinant human thyrotropin isoforms produced by Chinese hamster ovary cells: the role of sialylation and sulfation in thyrotropin bioactivity. Endocrinology 133: 1490–1503.
Igarashi, S., Minegishi, T., Nakamura, K., Nakamura, M., Tano, M., Miyamoto, K., et al. 1994. Functional expression of recombinant human luteinizing hormone/human choriogonadotropin receptor. Biochem. Biophys. Res. Commun. 201: 248–256.
Ascoli, M. 1981. Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptors and steroidogenic responses. Endocrinology 108: 88–95
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Szkudlinski, M., Teh, N., Grossmann, M. et al. Engineering human glycoprotein hormone superactive analogues. Nat Biotechnol 14, 1257–1263 (1996). https://doi.org/10.1038/nbt1096-1257
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nbt1096-1257
This article is cited by
-
Allosteric modulators of glycoprotein hormone receptors: discovery and therapeutic potential
Endocrine (2008)
-
Single chain human chorionic gonadotropin, hCGαβ: Effects of mutations in the α subunit on structure and bioactivity
Glycoconjugate Journal (2006)
-
Structure of human follicle-stimulating hormone in complex with its receptor
Nature (2005)