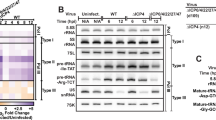
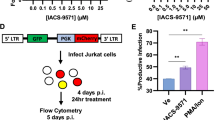
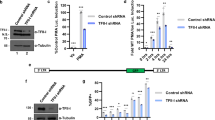
Abstract
Here we demonstrate that an inducible anti-HIV short hairpin RNA (shRNA) expressed from a Pol II promoter inhibits HIV-1 gene expression in mammalian cells. Our strategy is based on a promoter system in which the HIV-1 LTR is fused to the Drosophila hsp70 minimal heat shock promoter. This system is inducible by HIV-1 TAT, which functions in a negative feedback loop to activate transcription of an shRNA directed against HIV-1 rev. Upon induction the shRNA is processed to an siRNA that guides inhibition of HIV replication in cultured T-lymphocytes and hematopoietic stem cell–derived monocytes. The fusion promoter system may be safer than drug-inducible systems for shRNA-mediated gene therapy against HIV as the shRNAs are only expressed following HIV infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Grishok, A. et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106, 23–34 (2001).
Hutvagner, G. et al. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293, 834–838 (2001).
Wianny, F. & Zernica-Goetz, M. Specific interference with gene function by double stranded RNA in early mouse development. Nat. Cell Biol. 2, 70–75 (2000).
Kennerdell, J. & Carthew, R. Use of dsRNA-mediated genetic interference to demonstrate that frizzed and frizzled 2 act in the wingless pathway. Cell 95, 1017–1026 (1998).
Fire, A. et al. Potent and specific genetic interference by double stranded RNA in Caenorhabditis elegans . Nature 391, 806–811 (1998).
Lipardi, C., Wei, Q. & Patterson, B. RNAi as randomly degraded PCR. siRNA primers convert mRNA into dsRNAs that are degraded to generate new siRNAs. Cell 107, 297–307 (2001).
Sijen, T. et al. On the role of RNA amplification in dsRNA –triggered gene silencing. Cell 107, 465–476 (2001).
Caplan, N.J., Parrish, S., Imani, F., Fire, A. & Morgan, R.A. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc. Natl. Acad. Sci. USA 98, 9742–9747 (2001).
Elbashir, S.M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Brummelkamp, T.R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002).
Lee, N.S. et al. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat. Biotechnol. 20, 500–505 (2002).
Miyagishi, M. & Taira, K. U6 promoter-driven siRNAs with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nat. Biotechnol. 20, 497–500 (2002).
Xia, H., Paulson, H. & Davidson, BL. siRNA-mediated gene silencing in vitro and in vivo . Nat. Biotechnol. 20, 1006–1010 (2002).
An, D. et al. Efficient lentiviral vectors for short hairpin RNA delivery into human cells. Hum. Gene Ther. 14, 1207–1212 (2003).
Yi, R., Qin, Y., Macara, I. & Cullen, B. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 17, 3011–3016 (2003).
Lee, H. et al. DNA sequence requirements for generating paused polymerase at the start of hsp70. Genes Dev. 6, 284–295 (1992).
Dropulic, B., Hermankova, M. & Pitha, P. A conditionally replicating HIV-1 vector interferes with wild-type HIV-1 replication and spread. Proc. Natl. Acad. Sci. USA 93, 11103–11108 (1996).
Paik, S. et al. Defective HIV-1 provirus encoding a multi-target ribozyme inhibits accumulation of spliced and unspliced HIV-1 mRNAs, reduces infectivity of viral progeny, and protects the cells from pathogenesis. Hum. Gene Ther. 8, 1115–1123 (1997).
Karn, J. Tackling Tat. J. Mol. Biol. 293, 235–254 (1999).
Kobor, M. & Greenblatt, J. Regulation of transcription elongation by phosphorylation. Biochim. Biophys. Acta 1577, 261–275 (2002).
Brand, A. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993).
No, D., Yao, T. & Evans, R. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. USA 93, 3346–3351 (1996).
Krumm, A., Meulia, T. & Groudine, M. Common mechanisms for the control of eukaryotic transcriptional elongation. Bioessays 15, 659–665 (1993).
Kretz-Remy, C. & Arrigo, A. The kinetics of HIV-1 long terminal repeat transcriptional activation resemble those of hsp70 promoter in heat-shock treated HeLa cells. FEBS Lett. 351, 191–196 (1994).
Palangat, M., Meier, T., Keene, R. & Landick, R. Transcriptional pausing at +62 of the HIV-1 nascent RNA modulates formation of the TAR RNA structure. Mol. Cell 1, 1033–1042 (1998).
Mason, P. & Lis, J. Cooperative and competitive protein interactions at the hsp70 promoter. J. Biol. Chem. 272, 33227–33233 (1997).
Hsu, M. et al. Human fatty acid synthase gene: evidence for the presence of two promoters and their functional interaction. J. Biol. Chem. 271, 13584–13592 (1996).
Han, P., Brown, R. & Barsoum, J. Transactivation of heterologous promoters by HIV-1 Tat. Nucleic Acids Res. 19, 7225–7229 (1991).
Southgate, C. & Green, M. The HIV-1 tat protein activates transcription from an upstream DNA binding site: implications for tat function. Genes Dev. 5, 2496–2507 (1991).
Wiznerowicz, M. & Trono, D. Conditional suppression of cellular genes: lentivirus vector-mediated drug-inducible RNA interference. J. Virol. 77, 8957–8961 (2003).
Matsukura, S., Jones, P. & Takai, D. Establishment of conditional vectors for hairpin siRNA knockdowns. Nucleic Acids Res. 31, e77 (2003).
Van De Wetering, M. et al. Specific inhibition of gene expression using a stably integrated, inducible small-interfering-RNA vector. EMBO Rep. 4, 609–615 (2003).
Moss, E. & Taylor, J. Small-interfering RNAs in the radar of the interferon system. Nat. Cell Biol. 5, 771–772 (2003).
Sledz, C. et al. Activation of the interferon system by short-interfering RNAs. Nat. Cell Biol. 5, 834–839 (2003).
Bridge, A. et al. Induction of an interferon response by RNAi vectors in mammalian cells. Nat. Genet. 34, 263–264 (2003).
Gasmi, M. et al. Requirements for efficient production and transduction of human immunodeficiency virus type 1-based vectors. J. Virol. 73, 1828–1834 (1999).
Graham, F. & van der Eb, A. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52, 456–467 (1973).
Acknowledgements
This research was supported by National Institutes of Health grants AI 29329, AI42552 and HL074704.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Transcription occurs from both, HIV-1 LTR and mhsp70 TATA boxes. (PDF 79 kb)
Supplementary Fig. 2
Partial processing of shRNA loop. (PDF 88 kb)
Rights and permissions
About this article
Cite this article
Unwalla, H., Li, MJ., Kim, J. et al. Negative feedback inhibition of HIV-1 by TAT-inducible expression of siRNA. Nat Biotechnol 22, 1573–1578 (2004). https://doi.org/10.1038/nbt1040
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt1040
This article is cited by
-
A recombinant adenoviral vector with a specific tropism to CD4-positive cells: a new tool for HIV-1 inhibition
Drug Delivery and Translational Research (2022)
-
Chemically Modified Oligonucleotides Modulate an Epigenetically Varied and Transient Form of Transcription Silencing of HIV-1 in Human Cells
Molecular Therapy - Nucleic Acids (2012)
-
A dual function TAR Decoy serves as an anti-HIV siRNA delivery vehicle
Virology Journal (2010)
-
Short hairpin RNA-expressing oncolytic adenovirus-mediated inhibition of IL-8: effects on antiangiogenesis and tumor growth inhibition
Gene Therapy (2008)
-
Use of A U16 snoRNA-containing Ribozyme Library to Identify Ribozyme Targets in HIV-1
Molecular Therapy (2008)